Titanium tetrachloride
Upon contact with humid air, it forms thick clouds of titanium dioxide (TiO2) and hydrochloric acid, a reaction that was formerly exploited for use in smoke machines.
The tetrahedral structure for TiCl4 is consistent with its description as a d0 metal center (Ti4+) surrounded by four identical ligands.
TiCl4 is produced by the chloride process, which involves the reduction of titanium oxide ores, typically ilmenite (FeTiO3), with carbon under flowing chlorine at 900 °C.
This material, an impure form of TiO2, is derived from ilmenite by removal of iron, either using carbon reduction or extraction with sulfuric acid.
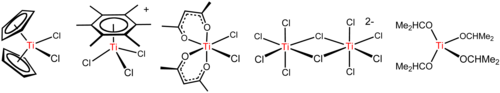
[16] Titanium tetrachloride is a versatile reagent that forms diverse derivatives including those illustrated below.
[17] A characteristic reaction of TiCl4 is its easy hydrolysis, signaled by the release of HCl vapors and titanium oxides and oxychlorides.
An illustrative reaction is the synthesis of tetrakis(dimethylamido)titanium Ti(N(CH3)2)4, a yellow, benzene-soluble liquid:[19] This molecule is tetrahedral, with planar nitrogen centers.
These reactions highlight the influence of electrostatics on the structures of compounds with highly ionic bonding.
This reaction illustrates the high Lewis acidity of the TiCl+3 entity, which is generated by abstraction of chloride from TiCl4 by AlCl3.
[12] TiCl4 finds occasional use in organic synthesis, capitalizing on its Lewis acidity, its oxophilicity, and the electron-transfer properties of its reduced titanium halides.
It is used in the Lewis acid catalysed aldol addition[22] Key to this application is the tendency of TiCl4 to activate aldehydes (RCHO) by formation of adducts such as (RCHO)TiCl4OC(H)R.[23] Hazards posed by titanium tetrachloride generally arise from its reaction with water that releases hydrochloric acid, which is severely corrosive itself and whose vapors are also extremely irritating.
TiCl4 is a strong Lewis acid, which exothermically forms adducts with even weak bases such as THF and water.