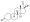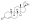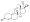Progestogen (medication)
A progestogen, also referred to as a progestagen, gestagen, or gestogen, is a type of medication which produces effects similar to those of the natural female sex hormone progesterone in the body.
[1] Side effects of progestogens include menstrual irregularities, headaches, nausea, breast tenderness, mood changes, acne, increased hair growth, and changes in liver protein production among others.
[1][2] Other side effects of progestogens may include an increased risk of breast cancer, cardiovascular disease, and blood clots.
[2] At high doses, progestogens can cause low sex hormone levels and associated side effects like sexual dysfunction and an increased risk of bone fractures.
[38] However, they produce various side effects, such as dyspnea, weight gain, vaginal bleeding, nausea, fluid retention, hypertension, thrombophlebitis, and thromboembolic complications.
[38][39] In addition, megestrol acetate has been found to be significantly inferior to aromatase inhibitors in the treatment of breast cancer, and in relation to this, progestogens have been moved down in the sequential therapy of the disease.
[1][2][46] Progestins with androgenic activity, namely 19-nortestosterone derivatives, can also cause acne, hirsutism, seborrhea, voice deepening, changes in liver protein production (e.g., decreased HDL cholesterol, sex hormone-binding globulin), increased appetite, and weight gain, among others.
[1][47] At high doses, due to their antigonadotropic effects, progestogens can cause low sex hormone levels and associated side effects like diminished secondary sexual characteristics, sexual dysfunction (e.g., reduced sex drive and erectile dysfunction), reversible infertility, reduced bone mineral density, and an increased risk of bone fractures, both in men and in premenopausal women.
[51] The progestins assessed included depot medroxyprogesterone acetate, levonorgestrel-containing contraceptive implants and intrauterine devices, and progestogen-only birth control pills.
[51] Findings of large observational studies are mixed due to prominent confounding factors, but overall show no association of hormonal birth control with depression.
[48][50][59] A 2016 systematic review found based on limited evidence from 6 studies that hormonal birth control, including combined birth control pills, depot medroxyprogesterone acetate, and levonorgestrel-containing intrauterine devices, was not associated with worse outcomes compared to non-use in women with depressive or bipolar disorders.
[79][80] Progestogens when used by themselves at typical clinical dosages, for instance in progestogen-only birth control, do not affect coagulation[81][82][83][84][75][77] and are not generally associated with a higher risk of venous thromboembolism (VTE).
[85][86][87][88] An exception is medroxyprogesterone acetate as a progestogen-only injectable contraceptive, which has been associated with a 2- to 4-fold increase in risk of VTE relative to other progestogens and non-use.
[89][90][91][92][93][94][88] The reasons for this are unknown, but the observations might be a statistical artifact of preferential prescription of depot medroxyprogesterone acetate to women at risk for VTE.
[90] Alternatively, medroxyprogesterone acetate may be an exception among progestogens in terms of influence on VTE risk,[88][92][81][94] possibly due to its partial glucocorticoid activity.
[103][104][105][106][107] The risk of VTE is increased by about 2-fold or less with such regimens in menopausal hormone therapy and by 2- to 4-fold with combined birth control pills containing ethinylestradiol, both relative to non-use.
[125][126][127] Combined birth control pills containing different progestins result in SHBG levels that are increased 1.5- to 2-fold with levonorgestrel, 2.5- to 4-fold with desogestrel and gestodene, 3.5- to 4-fold with drospirenone and dienogest, and 4- to 5-fold with cyproterone acetate.
[129][130][131][132][133] Estradiol-containing combined birth control pills, like estradiol valerate/dienogest and estradiol/nomegestrol acetate, and high-dose parenteral polyestradiol phosphate therapy have both been found to increase SHBG levels by about 1.5-fold.
[81][134][132][131] Hormone therapy with high-dose ethinylestradiol and cyproterone acetate in transgender women has been associated with a 20- to 45-fold higher risk of VTE relative to non-use.
[118][143][144][145][146][147] A 2015 Cochrane review provided strong evidence that the treatment of post-menopausal women with hormone therapy for cardiovascular disease had little if any effect and increased the risk of stroke and venous thromboembolic events.
[148] It is thought that androgenic progestins like medroxyprogesterone acetate and norethisterone may antagonize the beneficial effects of estrogens on biomarkers of cardiovascular health (e.g., favorable lipid profile changes).
[156][152][151] Some research has found that oral progesterone and dydrogesterone with short-term use (<5 years) may be associated with lower risk of breast cancer relative to other progestins.
[152][151][118][156] In the long-term however (>5 years), oral progesterone and dydrogesterone have been associated with significantly increased breast cancer risk similarly to other progestogens.
[151][152] A nationwide observational study found that transfeminine hormone therapy with estrogen plus high-dose cyproterone acetate was associated with a 46-fold increased risk of breast cancer in transgender women relative to the expected incidence for cisgender men.
[1] By activating PRs in the hypothalamus and pituitary gland, progestogens suppress the secretion of gonadotropins and thereby function as antigonadotropins at sufficiently high doses.
[258][1] Certain progestogens, including progesterone, drospirenone, and gestodene, as well as to a lesser extent dydrogesterone and trimegestone, have varying degrees of antimineralocorticoid activity.
[1] Progestins with potent antimineralocorticoid activity like drospirenone may have properties more similar to those of natural progesterone, such as counteraction of cyclical estrogen-induced sodium and fluid retention, edema, and associated weight gain; lowered blood pressure; and possibly improved cardiovascular health.
[265] Certain progestins have been found to stimulate the proliferation of MCF-7 breast cancer cells in vitro, an action that is independent of the classical PRs and is instead mediated via the progesterone receptor membrane component-1 (PGRMC1).
[266][267] It is unclear whether these findings may explain the different risks of breast cancer observed with progesterone, dydrogesterone, and other progestins such as medroxyprogesterone acetate and norethisterone in clinical studies.
[283][284][285][286][287] Noretynodrel, an isomer of norethisterone, was synthesized in 1952 by Frank B. Colton at Searle in Skokie, Illinois and used as the progestin in Enovid, marketed in the U.S. in 1957 and approved as the first oral contraceptive in 1960.