Programmed cell death
[citation needed] The first insight into the mechanism came from studying BCL2, the product of a putative oncogene activated by chromosome translocations often found in follicular lymphoma.
This trend has been highlighted with the award of the 2002 Nobel Prize in Physiology or Medicine to Sydney Brenner (United Kingdom), H. Robert Horvitz (US) and John E. Sulston (UK).
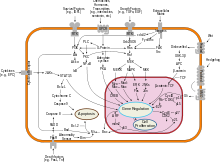
It is also becoming clear that mitosis and apoptosis are toggled or linked in some way and that the balance achieved depends on signals received from appropriate growth or survival factors.
These proteins are found in a majority of higher mammals as they are able to pierce the mitochondrial outer membrane - making them an integral part of mediating cell death by apoptosis.
[15] Macroautophagy, often referred to as autophagy, is a catabolic process that results in the autophagosomic-lysosomal degradation of bulk cytoplasmic contents, abnormal protein aggregates, and excess or damaged organelles.
[citation needed] Autophagy is generally activated by conditions of nutrient deprivation but has also been associated with physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection and cancer.
[22] Pyroptosis, an inflammatory type of cell death, is uniquely mediated by caspase 1, an enzyme not involved in apoptosis, in response to infection by certain microorganisms.
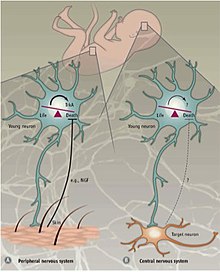
Common types of atrophic factors are:[23] The initial expansion of the developing nervous system is counterbalanced by the removal of neurons and their processes.
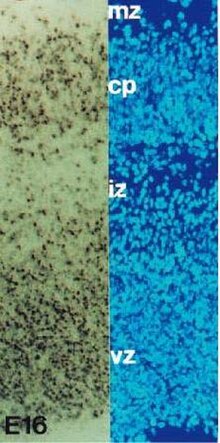
[28] This process of cell death has been identified in the germinal areas of the cerebral cortex, cerebellum, thalamus, brainstem, and spinal cord among other regions.
[27] Another theory proposes that developmental PCD in the nervous system occurs in order to correct for errors in neurons that have migrated ectopically, innervated incorrect targets, or have axons that have gone awry during path finding.
[30] It is possible that PCD during the development of the nervous system serves different functions determined by the developmental stage, cell type, and even species.
[31] It postulates that in order to ensure optimal innervation of targets, a surplus of neurons is first produced which then compete for limited quantities of protective neurotrophic factors and only a fraction survive while others die by programmed cell death.
[32] The underlying idea that target cells secrete attractive or inducing factors and that their growth cones have a chemotactic sensitivity was first put forth by Santiago Ramon y Cajal in 1892.
[33] Cajal presented the idea as an explanation for the "intelligent force" axons appear to take when finding their target but admitted that he had no empirical data.
[citation needed] Programmed cell death in the CNS is not dependent on external growth factors but instead relies on intrinsically derived cues.
[39] Together these findings indicate that programmed cell death in the CNS partly exploits Bax-mediated signaling and is independent of BDNF and the environment.
The absence or reduction of PCD can cause serious anatomical malformations but can also result in minimal consequences depending on the gene targeted, neuronal population, and stage of development.
[27] Excess progenitor cell proliferation that leads to gross brain abnormalities is often lethal, as seen in caspase-3 or caspase-9 knockout mice which develop exencephaly in the forebrain.
For example, mice overexpressing Bcl-2 have generally normal motor skills and vision and only show impairment in complex behaviors such as learning and anxiety.
[56] The similarity of the asymmetric cell death mechanism in the nematode and the leech indicates that PCD may have an evolutionary significance in the development of the nervous system.
[59] Furthermore, sex targeted cell death leads to different neuronal innervation of specific organs in males and females.
Extensive studies performed on various vertebrates show that PCD of neurons and glia occurs in most parts of the nervous system during development.
[65] Janneke Balk and Christopher J. Leaver, of the Department of Plant Sciences, University of Oxford, carried out research on mutations in the mitochondrial genome of sun-flower cells.
The researchers, Steven G. Thomas and Vernonica E. Franklin-Tong, also found that the response involves rapid inhibition of pollen-tube growth, followed by PCD.
[citation needed] D. discoideum is a slime mold, part of a branch that might have emerged from eukaryotic ancestors about a billion years before the present.
[73][74] Biologists had long suspected that mitochondria originated from bacteria that had been incorporated as endosymbionts ("living together inside") of larger eukaryotic cells.
Most of the time, invading bacteria are destroyed by the white blood cells; however, it is not uncommon for the chemical warfare waged by prokaryotes to succeed, with the consequence known as infection by its resulting damage.
[citation needed] One of these rare evolutionary events, about two billion years before the present, made it possible for certain eukaryotes and energy-producing prokaryotes to coexist and mutually benefit from their symbiosis.
[78] to cells (such as feedback from neighbors, stress or DNA damage), mitochondria release caspase activators that trigger the cell-death-inducing biochemical cascade.
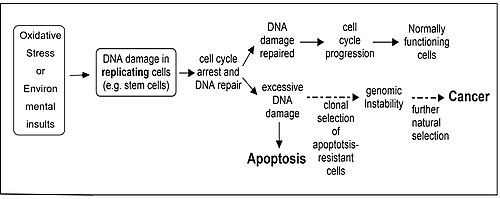
[citation needed] Repair of DNA damages and apoptosis are two enzymatic processes essential for maintaining genome integrity in humans.