Protein kinase R
[9] Binding to dsRNA is believed to activate PKR by inducing dimerization of the kinase domains and subsequent auto-phosphorylation reactions.
[12][13] Some research suggests that PKR can be stimulated by heat shock proteins, heparin, growth factors, bacterial infection, pro-inflammatory cytokines, reactive oxygen species, DNA damage, mechanical stress, and excess nutrient intake.
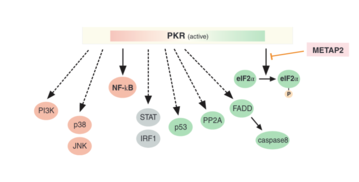
[13] PKR also has pro-inflammatory functions, as it can mediate the activation of the transcription factor NF-kB, by phosphorylating its inhibitory subunit, IkB.
[13] This leads to the expression of adhesion molecules and transcription factors that activate them, which induce inflammation responses such as the secretion of pro-inflammatory cytokines.
[16][17] Via the JNK signaling pathway, PKR also plays a role in insulin resistance, diabetes, and obesity by phosphorylating IRS1.
[18] Inhibiting PKR in mice led to lower inflammation in adipose tissues, increased sensitivity to insulin, and amelioration of diabetic symptoms.
[24] Activated PKR was specifically found in the cytoplasm and nucleus, as well as co-localized with neuronal apoptotic markers.
[25] Further studies have assessed the levels of PKR in blood and cerebrospinal fluid (CSF) of AD patients and controls.
The result of an analysis of the concentrations of total and phosphorylated PKR (pPKR) in peripheral blood mononuclear cells (PBMCs) in 23 AD patients and 19 control individuals showed statistically significant increased levels of the ratio of phosphorylated PKR/PKR in AD patients compared with controls.
A study found that "total PKR and pPKR concentrations were elevated in AD and amnestic mild cognitive impairment subjects with a pPKR value (optical density units) discriminating AD patients from control subjects with a sensitivity of 91.1% and a specificity of 94.3%.
Viral infection such as herpes simplex virus (HSV) or oxidative stress can both increase BACE1 expression through activation of PKR-eIF2a pathway.
[29] In addition, the increased activity of BACE1 could also lead to β-cleaved carboxy-terminal fragment of β-Amyloid precursor protein (APP-βCTF) induced dysfunction of endosomes in AD.
[32] Glycogen synthase kinase 3β (GSK-3β) is responsible for tau phosphorylation and controls several cellular functions including apoptosis.
[33] PKR also mediates ethanol-induced protein synthesis inhibition and apoptosis which is linked to fetal alcohol syndrome.