Protein phosphatase 1
PP1 has been found to be important in the control of glycogen metabolism, muscle contraction, cell progression, neuronal activities, splicing of RNA, mitosis,[1] cell division, apoptosis, protein synthesis, and regulation of membrane receptors and channels.
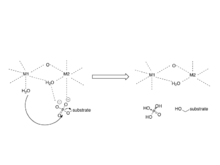
The interaction of the three β-sheets of the β-sandwich creates a channel for catalytic activity, as it is the site of coordination of metal ions.
[6] These metal ions have been identified as Mn and Fe and their coordination is provided by three histidines, two aspartic acids, and one asparagine.
[7] The mechanism involves two metal ions binding and activating water, which initiates a nucleophilic attack on the phosphorus atom.
[9] Microcystin is a liver toxin produced by blue-green algae and contains a cyclic heptapeptide structure that interacts with three distinct regions of the surface of the catalytic subunit of PP1.
[11] PP1 plays a crucial role in the regulation of blood glucose levels in the liver and glycogen metabolism.
Also, when other substrates become phosphorylated by protein kinase A, they can bind to the catalytic subunit of PP1 and directly inhibit it.
A 2019 study by researchers at Tsinghua, Fudan and the University of the Chinese Academy of Sciences demonstrated in both cell culture experiments and in PPP1R3G-knockdown mice that Akt (protein kinase B) directly phosphorylates Protein phosphatase 1 regulatory subunit 3G (PPP1R3G), which then binds to the PP1 complex, activating its phosphatase activity.
Researchers at Howard University showed that Tat protein targets PP1 to the nucleus and the consequent interaction is important for HIV-1 transcription.
EIF-2A is required for translation so by shutting down eIF-2A, the cell prevents the virus from hijacking its own protein-making machinery.
Herpesviruses in turn evolved ICP34.5 to defeat the defense; ICP34.5 activates protein phosphatase-1A which dephosphorylates eIF-2A, allowing translation to occur again.