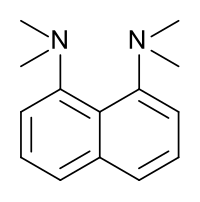
1,8-Bis(dimethylamino)naphthalene
With a pKa of 12.34[4] for its conjugate acid in aqueous solution, 1,8-bis(dimethylamino)naphthalene is one of the strongest organic bases.
The high basicity is attributed to the relief of strain upon protonation and/or the strong interaction between the nitrogen lone pairs.
[4] Proton sponge also exhibits a very high affinity for boron and is capable of displacing hydride from borane to form a boronium–borohydride ion pair.
1,8-bis(hexamethyltriaminophosphazenyl)naphthalene or HMPN[7] is prepared from 1,8-diaminonaphthalene by reaction with tris(dimethylamino)bromophosphonium bromide in the presence of triethylamine.
The aromatization of an additional ring in 4,12-Dihydrogen-4,8,12-triazatriangulene is utilized by Al-Yassiri and Puchta to get a representative for a new class of Δ-shaped proton sponges.