Quantum heat engines and refrigerators
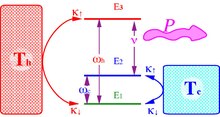
Quantum refrigerators share the structure of quantum heat engines with the purpose of pumping heat from a cold to a hot bath consuming power first suggested by Geusic, Schulz-DuBois, De Grasse and Scovil.
The relation between the quantum amplifier and the Carnot efficiency was first pointed out by Scovil and Schultz-DuBois:[1] Reversing the operation driving heat from the cold bath to the hot bath by consuming power constitutes a refrigerator.
Continuous devices include solar cells converting solar radiation to electrical power, thermoelectric where the output is current and lasers where the output power is coherent light.
A stroke is time segment in which a certain operation takes place (e.g. thermalization, or work extraction).
[12] In both types the quantum description allows to obtain equation of motion for the working medium and the heat flow from the reservoirs.
The only quantum effect can be found at low temperatures where the unit of energy of the device becomes
At high temperature and for the harmonic working medium the efficiency at maximum power becomes
[17] For shorter cycle times the working medium cannot follow adiabatically the change in the external parameter.
Surprisingly the dynamics leading to friction is quantized meaning that frictionless solutions to the adiabatic expansion/compression can be found in finite time.
[18] [19] As a result, optimization has to be carried out only with respect to the time allocated to heat transport.
The cycle is composed of a first stroke of partial equilibration of the two qubits with the hot and cold bath in parallel.
The swap operation is generated by a unitary transformation which preserves the entropy as a result it is a pure power stroke.
[9] The models differ by the choice of their working substance and heat source and sink.
Externally driven two-level,[24] three level[25] four-level[26][27] and coupled harmonic oscillators[28] have been studied.
The periodic driving splits the energy level structure of the working medium.
This splitting allows the two level engine to couple selectively to the hot and cold baths and produce power.
On the other hand, ignoring this splitting in the derivation of the equation of motion will violate the second law of thermodynamics.
While the intra cycle dynamics in the equivalence regime is very different in different engine types, when the cycle is completed they all turn out to provide the same amount of work and consume the same amount of heat (hence they share the same efficiency as well).
For heat engines a reduced description of the dynamics of the working substance is sought, tracing out the hot and cold baths.
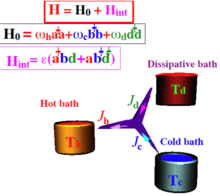
is the generator of dissipative dynamics which includes the heat transport terms from the baths.
Using this construction, the total change in energy of the sub-system becomes: leading to the dynamical version of the first law of thermodynamics:[6] The rate of entropy production becomes: The global structure of quantum mechanics is reflected in the derivation of the reduced description.
A derivation which is consistent with the laws of thermodynamics is based on the weak coupling limit.
can take the form of the Gorini-Kossakowski-Sudarshan-Lindblad (GKS-L) Markovian generator or also known just as Lindblad equation .
[35] [36][37] The absorption refrigerator is of unique importance in setting an autonomous quantum device.
In addition, in steady state the entropy is only generated in the baths, leading to the second law of thermodynamics: This version of the second-law is a generalisation of the statement of Clausius theorem; heat does not flow spontaneously from cold to hot bodies.
An energy current with no accompanying entropy production is equivalent to generating pure power:
There are seemingly two independent formulations of the third law of thermodynamics both originally were stated by Walther Nernst.
The second formulation, known as the unattainability principle can be rephrased as;[42] The dynamics of the cooling process is governed by the equation where
then the bath is cooled to zero temperature in a finite time, which implies a violation of the third law.
It is apparent from the last equation, that the unattainability principle is more restrictive than the Nernst heat theorem.