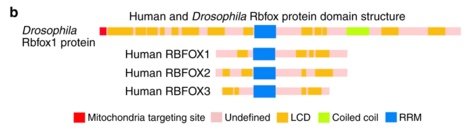
RBFOX1
[5] The RBFOX1 gene was first studied in Caenorhabditis elegans (nematodes), Drosophila melanogaster (fruit flies), and Danio rerio (zebrafish) with origins in embryology and development.
This refers to a lethal splicing event which causes an increase in the chromosomal X:A ratio; feminizing XO males.
In zebrafish, rbfox genes were identified as being essential for cardiac and skeletal muscle development, causing reduced heart rate and paralysis respectively in morphants.
HUD is human paraneoplastic encephalomyelitis antigen D whereas ELAV is Drosophila embryonic lethal abnormal visual protein.
Rbfox1, and the related protein Rbfox2, bind the consensus RNA sequence motif (U)GCAUG within introns to exert their functions as alternative splicing factors.
[12][13] The alternative splicing activity of RBFOX1 also aids in neuronal development specifically for CaV1.2 voltage-gated calcium channels and N-methyl-D-aspartate (NMDA receptors).
[14][15] The overall activity and molecular mechanism of alternative splicing mediation for RBFOX1 is not fully understood, but some qualities have been established in recent studies.
Conversely, for repression, both the RNA binding motif and carboxy terminal are required when tethered upstream of the alternative exon.
Possible proteins that aid in the inclusion or skipping process are not confirmed, though both hnRNPH and RALY have been shown to bind Rbfox1.
According to the DSM-5-TR, a diagnosis of Autism spectrum disorder requires at least two of the four restricted repetitive behaviors and all three verbal or nonverbal communication deficits.
Numerous autism spectrum disorder samples from cohorts and isolated autistic patients have been linked to de novo copy number variations of RBFOX1.
Similarly, whole transcriptome analysis of patients with autism spectrum disorder showcased a reduction of RBFOX1 and dysregulation of RBFOX1-dependent alternative splicing.
[13] While epilepsy, episodes of recurrent seizures, is most notably a neurological disorder, there are some cases which link the disease to issues with neuronal development.
Though it is unknown the specifics of how RBFOX1 affects neuronal development, it has been shown in neural-specific mouse knockouts that synaptic transmission and increased membrane excitability occur, causing a predisposition to seizures.
Oftentimes, a diagnosis requires a series of tests, observations, and questionnaires with the patient proving at least six of the nine inattentive and at least six of the nine hyperactivity and impulsivity symptoms (according to the DSM-5).
[24] Because RBFOX1 has been noted to affect neuronal migration and synapse formation, there may be reasonable concern for its contribution to predisposition of ADHD.
[25] Schizophrenia is a disorder with both positive (delusions, hallucinations, and disorganized thought) and negative (povery of speech, social withdrawal, and flattened effect) symptoms.
Several mechanisms play into the manifestation of this disease including ion channel dysfunction, RNA toxicity, and proteotoxicity.
Rbfox1 is noted to be a possible contributor to spinocerebellar ataxia type 2 (SCA2), one of twelve dominant repeat expansion SCAs.
The polyglutamine spinocerebellar aAtaxias not only have RNA foci and proteinaceous inclusions, but also the misfolded proteins themselves seem to aggregate in neuronal nuclei.
[29] In genetically predisposed or aged humans, these systems lose efficiency and can no longer handle the accumulating amount of misfolded tau, causing tangles to form more often without a way of clearing.
According to genome wide association (GWA) data, moduleQTL (modQTL) RBFOX1 SNP may alter gene expression of microglia.