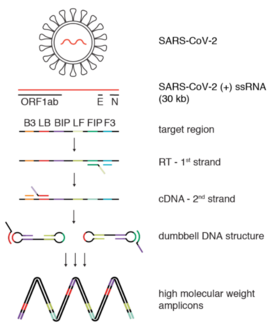
Reverse Transcription Loop-mediated Isothermal Amplification
[1] It combines LAMP[2] DNA-detection with reverse transcription, making cDNA from RNA before running the reaction.
[3] RT-LAMP does not require thermal cycles (unlike PCR) and is performed at a constant temperature between 60 and 65 °C.
The RT-LAMP technique is being supported as a cheaper and easier alternative to RT-PCR for the early diagnostics of people that are infectious for COVID-19.
Two of them are inner primers (FIP and BIP), which serve as base for the Bst enzyme copy the template into a new DNA.
The DNA polymerase and the FIP or BIP primers keep amplifying this strand and the LAMP-reaction product is extended.
Once this cycle has begun, the strand undergoes self-primed DNA synthesis during the elongation stage of the amplification process.
The sample is mixed with the primers, reverse transcriptase and DNA polymerase and the reaction takes place under a constant temperature.
PCR requires thermocycling; RT-LAMP does not, making it more time efficient and very cost effective.
[3] This inexpensive and streamlined method can be more readily used in developing countries that do not have access to high tech laboratories.