Radioactive displacement law of Fajans and Soddy
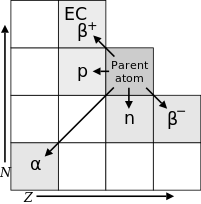
The law of radioactive displacements, also known as Fajans's and Soddy's law, in radiochemistry and nuclear physics, is a rule governing the transmutation of elements during radioactive decay.
It is named after Frederick Soddy and Kazimierz Fajans, who independently arrived at it at about the same time in 1913.
[1][2] The law describes which chemical element and isotope is created during the particular type of radioactive decay: