Noble gas
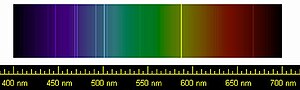
Under standard conditions, the first six of these elements are odorless, colorless, monatomic gases with very low chemical reactivity and cryogenic boiling points.
The seventh member of group 18 is oganesson, an unstable synthetic element whose chemistry is still uncertain because only five very short-lived atoms (t1/2 = 0.69 ms) have ever been synthesized (as of 2020[update][3]).
[6] Rare gases is another term that was used,[7] but this is also inaccurate because argon forms a fairly considerable part (0.94% by volume, 1.3% by mass) of the Earth's atmosphere due to decay of radioactive potassium-40.
Before them, in 1784, the English chemist and physicist Henry Cavendish had discovered that air contains a small proportion of a substance less reactive than nitrogen.
Along with Scottish scientist William Ramsay at University College, London, Lord Rayleigh theorized that the nitrogen extracted from air was mixed with another gas, leading to an experiment that successfully isolated a new element, argon, from the Greek word ἀργός (argós, "idle" or "lazy").
In 1898, he discovered the elements krypton, neon, and xenon, and named them after the Greek words κρυπτός (kryptós, "hidden"), νέος (néos, "new"), and ξένος (ksénos, "stranger"), respectively.
[13] Rayleigh and Ramsay received the 1904 Nobel Prizes in Physics and in Chemistry, respectively, for their discovery of the noble gases;[14][15] in the words of J. E. Cederblom, then president of the Royal Swedish Academy of Sciences, "the discovery of an entirely new group of elements, of which no single representative had been known with any certainty, is something utterly unique in the history of chemistry, being intrinsically an advance in science of peculiar significance".
In 1895, French chemist Henri Moissan attempted to form a reaction between fluorine, the most electronegative element, and argon, one of the noble gases, but failed.
Scientists were unable to prepare compounds of argon until the end of the 20th century, but these attempts helped to develop new theories of atomic structure.
[20] In October 2006, scientists from the Joint Institute for Nuclear Research and Lawrence Livermore National Laboratory successfully created synthetically oganesson, the seventh element in group 18,[21] by bombarding californium with calcium.
For example, the ionization potential decreases with an increasing radius because the valence electrons in the larger noble gases are farther away from the nucleus and are therefore not held as tightly together by the atom.
To do this, the nearest noble gas that precedes the element in question is written first, and then the electron configuration is continued from that point forward.
This causes some inconsistencies in trends across the table, and on those grounds some chemists have proposed that helium should be moved to group 2 to be with other s2 elements,[37][38][39] but this change has not generally been adopted.
[59] Because it is composed of the two most abundant elements in the universe, hydrogen and helium, it was believed to occur naturally in the interstellar medium, and it was finally detected in April 2019 using the airborne SOFIA telescope.
The essential condition for their formation is that the guest (noble gas) atoms must be of appropriate size to fit in the cavities of the host crystal lattice.
For instance, argon, krypton, and xenon form clathrates with hydroquinone, but helium and neon do not because they are too small or insufficiently polarizable to be retained.
In 1993, it was discovered that when C60, a spherical molecule consisting of 60 carbon atoms, is exposed to noble gases at high pressure, complexes such as He@C60 can be formed (the @ notation indicates He is contained inside C60 but not covalently bound to it).
[64] These compounds have found use in the study of the structure and reactivity of fullerenes by means of the nuclear magnetic resonance of the noble gas atom.
Due to its high radioactivity, radon presents a significant health hazard; it is implicated in an estimated 21,000 lung cancer deaths per year in the United States alone.
[80] Neon, argon, krypton, and xenon are obtained from air using the methods of liquefaction of gases, to convert elements to a liquid state, and fractional distillation, to separate mixtures into component parts.
Gases are absorbed by the blood and body tissues when under pressure like in scuba diving, which causes an anesthetic effect known as nitrogen narcosis.
[78] Xenon is used as an anesthetic because of its high solubility in lipids, which makes it more potent than the usual nitrous oxide, and because it is readily eliminated from the body, resulting in faster recovery.
Due to their inert nature and low abundances, change in the noble gas concentration and their isotopic ratios can be used to resolve and quantify the processes influencing their current signatures across geological settings.
Production of these two isotopes (36Ar and 38Ar) is negligible within the earth's crust, only limited concentrations of 38Ar can be produced by interaction between alpha particles from decay of 235,238U and 232Th and light elements (37Cl and 41K).
[115] This is largely due to a clear distinction of krypton isotope signature from various sources such as chondritic material, solar wind and cometary.
Krypton and xenon noble gases requires pristine, robust geochemical sampling protocol to avoid atmospheric contamination.
Noble gas measurements can be obtained from sources like volcanic vents, springs, and geothermal wells following specific sampling protocols.
Originally, scientists used magnetic sector mass spectrometry, which is time-consuming and has low sensitivity due to "peak jumping mode".
[126] Before analysis, sample preparation is essential due to the low abundance of noble gases, involving extraction, purification system.
[95] Extraction allows liberation of noble gases from their carrier (major phase; fluid or solid) while purification remove impurities and improve concentration per unit sample volume.


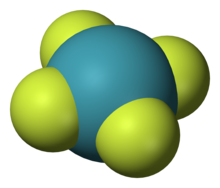
4 ), one of the first noble gas compounds to be discovered
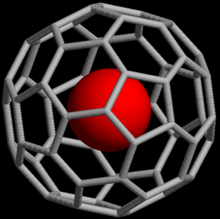

2 according to the 3-center-4-electron bond model




