Rotational spectroscopy
In the presence of an electrostatic field there is Stark splitting which allows molecular electric dipole moments to be determined.
An important application of rotational spectroscopy is in exploration of the chemical composition of the interstellar medium using radio telescopes.
Much of current understanding of the nature of weak molecular interactions such as van der Waals, hydrogen and halogen bonds has been established through rotational spectroscopy.
In connection with radio astronomy, the technique has a key role in exploration of the chemical composition of the interstellar medium.
Current projects in astrochemistry involve both laboratory microwave spectroscopy and observations made using modern radiotelescopes such as the Atacama Large Millimeter/submillimeter Array (ALMA).
[9][notes 4] A consequence of this rule is that no microwave spectrum can be observed for centrosymmetric linear molecules such as N2 (dinitrogen) or HCCH (ethyne), which are non-polar.
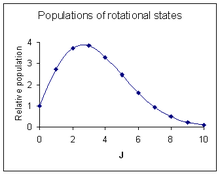
For all other molecules both Stokes and anti-Stokes lines[notes 5] can be observed and they have similar intensities due to the fact that many rotational states are thermally populated.
[14] The value ΔJ = 0 does not correspond to a molecular transition but rather to Rayleigh scattering in which the incident photon merely changes direction.
For example, the molecule methane is a spherical top but the asymmetric C-H stretching band shows rotational fine structure in the infrared spectrum, illustrated in rovibrational coupling.
A linear molecule lies on a single axis and each atom moves on the surface of a sphere around the centre of mass.
The two degrees of rotational freedom correspond to the spherical coordinates θ and φ which describe the direction of the molecular axis, and the quantum state is determined by two quantum numbers J and M. J defines the magnitude of the rotational angular momentum, and M its component about an axis fixed in space, such as an external electric or magnetic field.
The probability of a transition taking place is the most important factor influencing the intensity of an observed rotational line.
To account for this a centrifugal distortion correction term is added to the rotational energy levels of the diatomic molecule.
If anharmonicity is to be taken into account, terms in higher powers of J should be added to the expressions for the energy levels and line positions.
[24] With a first order correction for centrifugal distortion the transition wavenumbers become The term in DJK has the effect of removing degeneracy present in the rigid rotor approximation, with different K values.
For this reason far infrared spectrometers have to be freed of atmospheric water vapour either by purging with a dry gas or by evacuation.
[31] A similar removal of degeneracy will occur when a paramagnetic molecule is placed in a magnetic field, an instance of the Zeeman effect.
[33] The great majority of contemporary spectrometers use a mixture of commercially available and bespoke components which users integrate according to their particular needs.
Although rotational transitions can be found across a very broad region of the electromagnetic spectrum, fundamental physical constraints exist on the operational bandwidth of instrument components.
The instruments and operating principals described below are generally appropriate to microwave spectroscopy experiments conducted at frequencies between 6 and 24 GHz.
An important variation of the technique in which an alternating current is applied across electrodes within the absorption cell results in a modulation of the frequencies of rotational transitions.
[34] Subsequent experiments exploited powerful sources of microwaves such as the klystron, many of which were developed for radar during the Second World War.
[39] Balle, Campbell, Keenan and Flygare demonstrated that the FTMW technique can be applied within a "free space cell" comprising an evacuated chamber containing a Fabry-Perot cavity.
[40] This technique allows a sample to be probed only milliseconds after it undergoes rapid cooling to only a few kelvins in the throat of an expanding gas jet.
This was a revolutionary development because (i) cooling molecules to low temperatures concentrates the available population in the lowest rotational energy levels.
Coupled with benefits conferred by the use of a Fabry-Perot cavity, this brought a great enhancement in the sensitivity and resolution of spectrometers along with a reduction in the complexity of observed spectra; (ii) it became possible to isolate and study molecules that are very weakly bound because there is insufficient energy available for them to undergo fragmentation or chemical reaction at such low temperatures.
While the Fabry-Perot cavity of a Balle-Flygare FTMW spectrometer can typically be tuned into resonance at any frequency between 6 and 18 GHz, the bandwidth of individual measurements is restricted to about 1 MHz.
Pate at the University of Virginia[42] designed a spectrometer[43] which retains many advantages of the Balle-Flygare FT-MW spectrometer while innovating in (i) the use of a high speed (>4 GS/s) arbitrary waveform generator to generate a "chirped" microwave polarisation pulse that sweeps up to 12 GHz in frequency in less than a microsecond and (ii) the use of a high speed (>40 GS/s) oscilloscope to digitise and Fourier transform the molecular free induction decay.
The result is an instrument that allows the study of weakly bound molecules but which is able to exploit a measurement bandwidth (12 GHz) that is greatly enhanced compared with the Balle-Flygare FTMW spectrometer.
Modified versions of the original CP-FTMW spectrometer have been constructed by a number of groups in the United States, Canada and Europe.
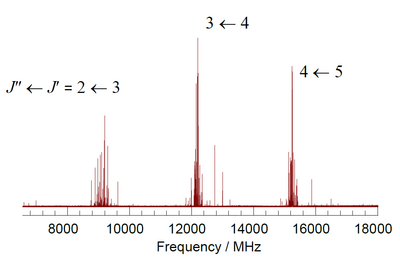
3 I . [ notes 1 ] Each rotational transition is labeled with the quantum numbers, J , of the final and initial states, and is extensively split by the effects of nuclear quadrupole coupling with the 127 I nucleus.