Rubber toughening
By "toughening" a polymer it is meant that the ability of the polymeric substance to absorb energy and plastically deform without fracture is increased.
[4] The effects of disperse rubber nanoparticles are complex and differ across amorphous and partly crystalline polymeric systems.
[5] Rubber particles toughen a system by a variety of mechanisms such as when particulates concentrate stress causing cavitation or initiation of dissipating crazes.
[7] It is difficult to state the specific effects of a given particle size or interfacial adhesion parameter due to numerous other confounding variables.
[6] The presence of a given failure mechanism is determined by many factors: those intrinsic to the continuous polymer phase,[6] and those that are extrinsic, pertaining to the stress, loading speed, and ambient conditions.
The addition of rubbery domains occurs via processes such as melt blending in a Rheomix mixer and atom-transfer radical-polymerization.
[4][8] Current research focuses on how optimizing the secondary phase composition and dispersion affects mechanical properties of the blend.
[9] Different theories describe how a dispersed rubber phase toughens a polymeric substance; most employ methods of dissipating energy throughout the matrix.
[5] Two key observations that went into the initial theory and subsequent expansion were as follows: (1) microcracks form voids over which styrene-butadiene copolymer fibrils form to prevent propagation, and (2) energy stored during elongation of toughened epoxies is released upon breaking of rubber particles.
Crazes start at the equator where principal strain is highest, propagate perpendicular to the stress, and end when they meet another particle.
If this force overcomes the surface adhesion between the rubber and polymer, debonding will occur, thereby diminishing the toughening effect associated with crazing.
[6] Shear yielding theory is one that, like matrix crazing, can account for a large fraction of the increase in energy absorption of a toughened polymer.
"[6] Shear yielding will result if rubber particles act as stress concentrators and initiate volume-expansion through crazing, debonding and cavitation, to halt the formation of cracks.
[6] Cavitation is common in epoxy resins and other craze resistant toughened polymers, and is prerequisite to shearing in Izod impact strength testing.
Engineers use an energy-balance approach to model how particle size and rubber modulus factors influence material toughness.
"[11] The energy-balancing model applies the physical properties of the whole material to describe the microscopic behavior during triaxial stress.
[10][11] The damage competition theory models the relative contributions of shear yielding and craze failure, when both are present.
Partly crystalline thermoplastics are tough and creep-prone because the amorphous regions surrounding the rigid crystals are afforded some mobility.
[13] In applications where high optical transparency is necessary, examples being poly(methyl methacrylate) and polycarbonate it is important to find a secondary phase that does not scatter light.
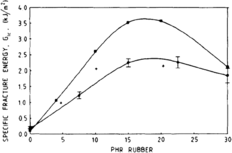
In one study, looking at PA6-EPDM blend, increasing the concentration of rubber up to 30 percent showed a negative linear relationship with the brittle-tough transition temperature, after which the toughness decreased.
At fixed rubber concentrations, one can find that an optimal particle size is a function of the entanglement density of the polymer matrix.
For impact strength testing on PMMA where failure occurs by shear-yielding, the optimum size of filler PBA-core PMMA-shell particle was shown in one case to be 250 nm.
In the three-point bend test, where failure is due to crazing, 2000 nm particles had the most significant toughening effect.
As a result, the continuous phase fails by mechanisms characteristic of the pure polymer, as if the rubber was not present.
As temperature rises further past the glass transition of the rubber phase, the impact strength of a rubber-polymer composite still dramatically increases as crack propagation requires additional energy input.
[13] This finding underlines how this field is constantly growing and more work can be done to better model the rubber toughening effect.
The polybutadiene rubber domains in the main styrene-acrylonitrile matrix act as a stop to crack propagation.
Developing PMMA compatible core-shell particles with low glass transition temperature while maintaining optical transparency is of interest to architects and car companies.
The alternative emulsion polymerization with styrene-butadiene-styrene or styrene-butadiene copolymers allows fine-tuned manipulation of particle size distribution.
[26] In order to study the fracture microstructure of HIPS in a transmission electron microscope it is necessary to stain one of the phases with a heavy metal, Osmium tetroxide for example.