Polybutadiene
Polybutadiene is typically crosslinked with sulphur, however, it has also been shown that it can be UV cured when bis-benzophenone additives are incorporated into the formulation.
Another 25% is used as an additive to improve the toughness (impact resistance) of plastics such as polystyrene and acrylonitrile butadiene styrene (ABS).
[2] Polybutadiene is also used to manufacture golf balls, various elastic objects and to coat or encapsulate electronic assemblies, offering high electrical resistivity.
[4][5] In 1926 he invented a process for manufacturing butadiene from ethanol, and in 1928, developed a method for producing polybutadiene using sodium as a catalyst.
The government of the Soviet Union strove to use polybutadiene as an alternative to natural rubber and built the first pilot plant in 1930,[6] using ethanol produced from potatoes.
The experiment was a success and in 1936 the Soviet Union built the world's first polybutadiene plant in which the butadiene was obtained from petroleum.
[7] Following Lebedev's work, other industrialized countries such as Germany and the United States developed polybutadiene and SBR as an alternative to natural rubber.
The leading manufacturers of tires and some petrochemical companies began to build polybutadiene plants on all inhabited continents; the boom lasted until the 1973 oil crisis.
In Germany, scientists from Bayer (at the time a part of the conglomerate IG Farben) reproduced Lebedev's processes of producing polybutadiene by using sodium as a catalyst.
[8][9] Although the Goodrich Corporation had successfully developed a process for producing polybutadiene in 1939,[10] the U.S. federal government opted for the use of Buna-S to develop its synthetic-rubber industry after its entry into the World War II,[6] using patents of IG Farben obtained via Standard Oil.
The following year, Firestone Tire and Rubber Company was first to produce low cis polybutadiene using butyllithium as a catalyst.
In 1965, the Japanese JSR Corporation developed its own low cis process and began licensing it during the next decade.
The 1973 oil crisis marked a halt to the growth of synthetic rubber production; the expansion of existing plants almost ceased for a few years.
The world's largest producer of polybutadiene, Bayer, went through major restructuring as it was troubled by financial losses; between 2002 and 2005 it closed its cobalt-polybutadiene plants in Sarnia (Canada) and Marl (Germany),[13] transferring their production to neodymium plants in Port Jérôme (France) and Orange (USA).
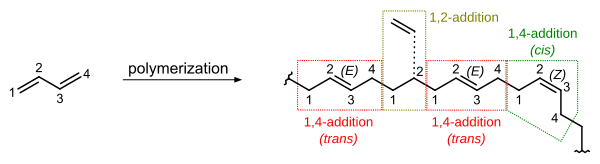
In terms of the connectivity of the polymer chain, butadiene can polymerize in three different ways, called cis, trans and vinyl.
It has been found that a substantial percentage of cis double bond configurations in the polymer will result in a material with flexible elastomer (rubber-like) qualities.
In free radical polymerization, both cis and trans double bonds will form in percentages that depend on temperature.
Polybutadiene can be produced with more than 90% trans using catalysts similar to those of high cis: neodymium, lanthanum, nickel.
The polybutadiene is used primarily in the sidewall of truck tires, this helps to improve fatigue to failure life due to the continuous flexing during run.
[29] About 25% of the produced polybutadiene is used to improve the mechanical properties of plastics, in particular of high-impact polystyrene (HIPS) and to a lesser extent acrylonitrile butadiene styrene (ABS).
Most golf balls are made of an elastic core of polybutadiene surrounded by a layer of a harder material.
First, polybutadiene is mixed with additives, then extruded, pressed using a calender and cut into pieces which are placed in a mold.