SDS-PAGE
SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) is a discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate proteins with molecular masses between 5 and 250 kDa.
[8] SDS acts as a surfactant, masking the protein's intrinsic charge and conferring them very similar charge-to-mass ratios.
Upon application of a constant electric field, the proteins migrate towards the anode, each with a different speed, depending on their mass.
[5] SDS is amphipathic in nature, which allows it to unfold both polar and nonpolar sections of protein structure.
Although the native, fully folded, SDS-resistant protein does not have sufficient stability in the presence of SDS, the chemical equilibrium of denaturation at room temperature occurs slowly.
Stable protein complexes are characterised not only by SDS resistance but also by stability against proteases and an increased biological half-life.
[11] Alternatively, polyacrylamide gel electrophoresis can also be performed with the cationic surfactants CTAB in a CTAB-PAGE,[12][13][14] or 16-BAC in a BAC-PAGE.
By adding the catalyst TEMED and the radical initiator ammonium persulfate (APS) the polymerisation is started.
[20] This gel system has a comparatively large separation range, which can be varied by using MES or MOPS in the running buffer.
For separation, the denatured samples are loaded onto a gel of polyacrylamide, which is placed in an electrophoresis buffer with suitable electrolytes.
Due to the relatively small molecule size of bromophenol blue, it migrates faster than proteins.
[39][40] In Coomassie staining, gel is fixed in a 50% ethanol 10% glacial acetic acid solution for 1 hr.
After protein staining and documentation of the banding pattern, the polyacrylamide gel can be dried for archival storage.
The drying frame consists of two parts, one of which serves as a base for a wet cellophane film to which the gel and a one percent glycerol solution are added.
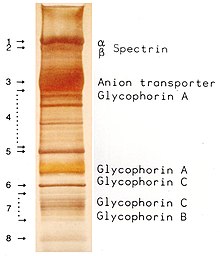
For a more accurate determination of the molecular weight, the relative migration distances of the individual protein bands are measured in the separating gel.
By comparison with the linear part of the generated graph or by a regression analysis, the molecular weight of an unknown protein can be determined by its relative mobility.
[47] Proteins with many basic amino acids (e. g. histones)[48] can lead to an overestimation of the molecular weight or even not migrate into the gel at all, because they move slower in the electrophoresis due to the positive charges or even to the opposite direction.
SDS-PAGE for proteinuria evaluates the levels of various serum proteins in the urine, e.g. Albumin, Alpha-2-macroglobulin and IgG.
Two-dimensional gel electrophoresis sequentially combines isoelectric focusing or BAC-PAGE with a SDS-PAGE.
For electrophoretic separation of larger protein complexes, agarose gel electrophoresis can be used, e.g. the SDD-AGE.
In 1948, Arne Tiselius was awarded the Nobel Prize in Chemistry for the discovery of the principle of electrophoresis as the migration of charged and dissolved atoms or molecules in an electric field.
[58] The use of a solid matrix (initially paper discs) in a zone electrophoresis improved the separation.
The discontinuous electrophoresis of 1964 by L. Ornstein and B. J. Davis made it possible to improve the separation by the stacking effect.
The denaturing effect of SDS in continuous polyacrylamide gels and the consequent improvement in resolution was first described in 1965 by David F. Summers in the working group of James E. Darnell to separate poliovirus proteins.
[60] The current variant of the SDS-PAGE was described in 1970 by Ulrich K. Laemmli and initially used to characterise the proteins in the head of bacteriophage T4.