Polyacrylamide gel electrophoresis
Acrylamide is soluble in water and upon addition of free-radical initiators it polymerizes resulting in formation of polyacrylamide.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) is a method of separating molecules based on the difference of their molecular weight.
By binding to the proteins the detergent destroys their secondary, tertiary and/or quaternary structure denaturing them and turning them into negatively charged linear polypeptide chains.
When subjected to an electric field in PAGE, the negatively charged polypeptide chains travel toward the anode with different mobility.
[4] By comparing the relative ratio of the distance traveled by each protein to the length of the gel (Rf) one can make conclusions about the relative molecular weight of the proteins, where the length of the gel is determined by the distance traveled by a small molecule like a tracking dye.
In most proteins, the binding of SDS to the polypeptide chains impart an even distribution of charge per unit mass, thereby resulting in a fractionation by approximate size during electrophoresis.
Native-PAGE keeps the oligomeric form intact and will show a band on the gel that is representative of the level of activity.
SDS-PAGE will denature and separate the oligomeric form into its monomers, showing bands that are representative of their molecular weights.
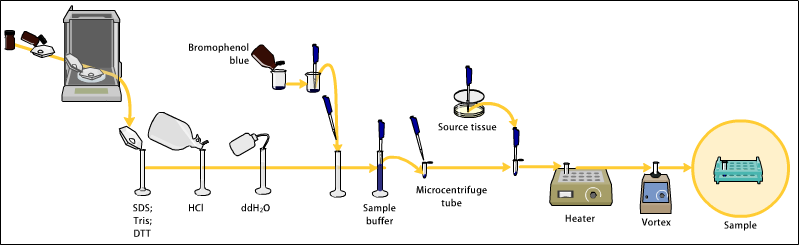
These may be biologically derived, for example from prokaryotic or eukaryotic cells, tissues, viruses, environmental samples, or purified proteins.
In the case of solid tissues or cells, these are often first broken down mechanically using a blender (for larger sample volumes), using a homogenizer (smaller volumes), by sonicator or by using cycling of high pressure, and a combination of biochemical and mechanical techniques – including various types of filtration and centrifugation – may be used to separate different cell compartments and organelles prior to electrophoresis.
The sample to analyze is optionally mixed with a chemical denaturant if so desired, usually SDS for proteins or urea for nucleic acids.
SDS is an anionic detergent that denatures secondary and non–disulfide–linked tertiary structures, and additionally applies a negative charge to each protein in proportion to its mass.
Urea breaks the hydrogen bonds between the base pairs of the nucleic acid, causing the constituent strands to separate.
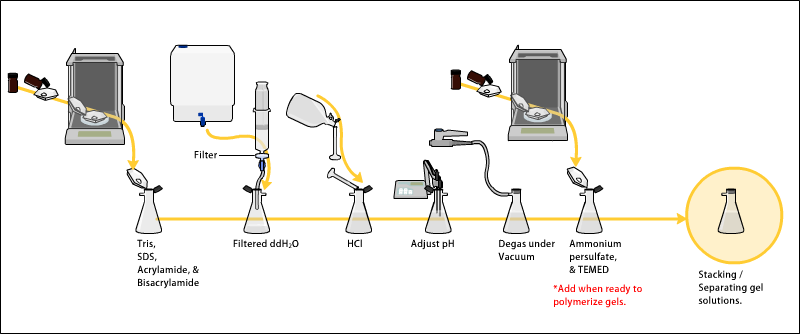
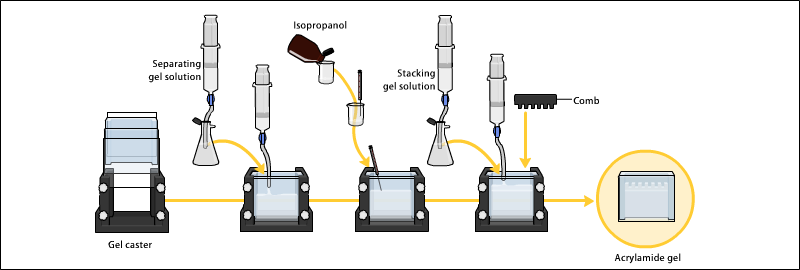
The gels typically consist of acrylamide, bisacrylamide, the optional denaturant (SDS or urea), and a buffer with an adjusted pH.
Smaller biomolecules travel farther down the gel, while larger ones remain closer to the point of origin.
[3]: 161–3 For analytical purposes, the relative mobility of biomolecules, Rf, the ratio of the distance the molecule traveled on the gel to the total travel distance of a tracking dye is plotted versus the molecular weight of the molecule (or sometimes the log of MW, or rather the Mr, molecular radius).
Such typically linear plots represent the standard markers or calibration curves that are widely used for the quantitative estimation of a variety of biomolecular sizes.
Additionally, the analysis of larger proteins ranging from 250,000 to 600,000 Da is also reported to be problematic due to the fact that such polypeptides move improperly in the normally used gel systems.
It is a synthetic, thermo-stable, transparent, strong, chemically relatively inert gel, and can be prepared with a wide range of average pore sizes.
This gel material can also withstand high voltage gradients, is amenable to various staining and destaining procedures, and can be digested to extract separated fractions or dried for autoradiography and permanent recording.
Additionally, stacking gels usually have a pH of 6.8, since the neutral glycine molecules allow for faster protein mobility.
Counterion balance the intrinsic charge of the buffer ion and also affect the electric field strength during electrophoresis.
Highly charged and mobile ions are often avoided in SDS-PAGE cathode buffers, but may be included in the gel itself, where it migrates ahead of the protein.
In applications such as DISC SDS-PAGE the pH values within the gel may vary to change the average charge of the counterions during the run to improve resolution.
[22] It is also essential to store acrylamide in a cool dark and dry place to reduce autopolymerisation and hydrolysis.
Adding SDS solves this problem, as it binds to and unfolds the protein, giving a near uniform negative charge along the length of the polypeptide.
Macromolecular structure is dependent on the net effect of these forces, therefore it follows that an increase in chaotropic solutes denatures macromolecules, Ammonium persulfate (APS) (N2H8S2O8; mW: 228.2) is a source of free radicals and is often used as an initiator for gel formation.
Tracking dye; as proteins and nucleic acids are mostly colorless, their progress through the gel during electrophoresis cannot be easily followed.
This dye is coloured at alkali and neutral pH and is a small negatively charged molecule that moves towards the anode.
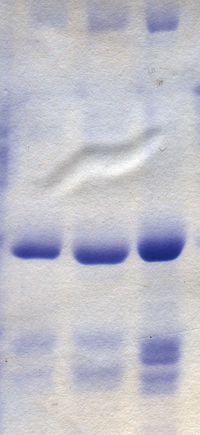
[25] Silver staining was introduced by Kerenyi and Gallyas as a sensitive procedure to detect trace amounts of proteins in gels.