Capillary electrophoresis
In the most common mode of CE, all ions, positive or negative, are pulled through the capillary in the same direction by electroosmotic flow.
[3] Capillary electrophoresis was first combined with mass spectrometry by Richard D. Smith and coworkers, and provides extremely high sensitivity for the analysis of very small sample sizes.
Certain aspects of the instrumentation (such as detection) are necessarily more complex than for a single-capillary system, but the fundamental principles of design and operation are similar to those shown in Figure 1.
For polyimide-coated capillaries, a segment of the coating is typically burned or scraped off to provide a bare window several millimeters long.
[4] This decrease is almost unnoticeable if a smooth aneurysm is produced in the wall of a capillary by heating and pressurization, as plug flow can be preserved.
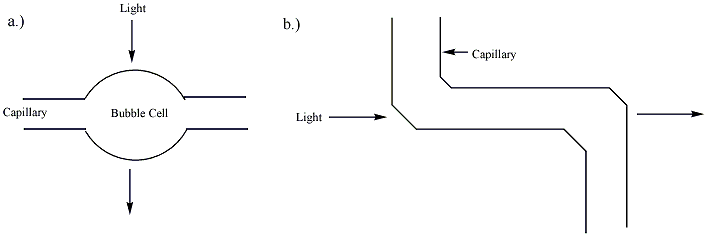
When used with a UV absorbance detector, the wider cross-section of the analyte in the cell allows for an illuminating beam twice as large, which reduces shot noise by a factor of two.
Together these two factors increase the sensitivity of Agilent Technologies's Bubble Cell CE Detector six times over that of one using a straight capillary.
Numerous labeling strategies are used to create fluorescent derivatives or conjugates of non-fluorescent molecules, including proteins and DNA.
[5][6] In order to obtain the identity of sample components, capillary electrophoresis can be directly coupled with mass spectrometers or surface-enhanced Raman spectroscopy (SERS).
This setup requires volatile buffer solutions, which will affect the range of separation modes that can be employed and the degree of resolution that can be achieved.
Analyte retention times can be translated into spatial distance by moving the SERS-active substrate at a constant rate during capillary electrophoresis.
[7] The separation of compounds by capillary electrophoresis is dependent on the differential migration of analytes in an applied electric field.
[4] Since only charged ions are affected by the electric field, neutral analytes are poorly separated by capillary electrophoresis.
The velocity of migration of an analyte in capillary electrophoresis will also depend upon the rate of electroosmotic flow (EOF) of the buffer solution.
[4] Electroosmotic flow is observed when an electric field is applied to a solution in a capillary that has fixed charges on its interior wall.
The mobile cation layer is pulled in the direction of the negatively charged cathode when an electric field is applied.
One of the most common approaches to suppressing EOF, reported by Stellan Hjertén in 1985, is to create a covalently attached layer of linear polyacrylamide.
[8] The silica surface of the capillary is first modified with a silane reagent bearing a polymerizable vinyl group (e.g. 3-methacryloxypropyltrimethoxysilane), followed by introduction of acrylamide monomer and a free radical initiator.
The acrylamide is polymerized in situ, forming long linear chains, some of which are covalently attached to the wall-bound silane reagent.
[9] For example, in capillary sequencing of DNA, the sieving polymer (typically polydimethylacrylamide) suppresses electroosmotic flow to very low levels.
At sufficiently high field strengths, this heating is strong enough that radial temperature gradients can develop within the capillary.
The onset of significant Joule heating can be determined by constructing an "Ohm's Law plot", wherein the current through the capillary is measured as a function of applied potential.
The best resolution is typically obtained at the maximum field strength for which Joule heating is insignificant (i.e. near the boundary between the linear and nonlinear regimes of the Ohm's Law plot).
[4] Besides diffusion and Joule heating (discussed above), factors that may decrease the resolution in capillary electrophoresis from the theoretical limits in the above equation include, but are not limited to, the finite widths of the injection plug and detection window; interactions between the analyte and the capillary wall; instrumental non-idealities such as a slight difference in height of the fluid reservoirs leading to siphoning; irregularities in the electric field due to, e.g., imperfectly cut capillary ends; depletion of buffering capacity in the reservoirs; and electrodispersion (when an analyte has higher conductivity than the background electrolyte).
[12] Identifying and minimizing the numerous sources of band broadening is key to successful method development in capillary electrophoresis, with the objective of approaching as close as possible to the ideal of diffusion-limited resolution.
These capillaries have excellent capabilities to dissipate heat, permitting much higher electric field strengths to be used than slab gel electrophoresis.
[14] A major use of CE by forensic biologists is typing of STR from biological samples to generate a profile from highly polymorphic genetic markers which differ between individuals.
Other emerging uses for CE include the detection of specific mRNA fragments to help identify the biological fluid or tissue origin of a forensic sample.
Micellar electrophoretic capillary chromatography (MECC) has been developed and applied to the analysis of inks extracted from paper.
[18] The use of ACE can provide specific details in binding, separation, and detection of analytes and is proven to be highly practical for studies in life sciences.