Scandium fluoride
This salt is slightly soluble in water but dissolves in the presence of excess fluoride to form the ScF63− anion.
[4] Scandium trifluoride exhibits the unusual property of negative thermal expansion, meaning it shrinks when heated.
[5] ScF3 exhibits this property from at least 10 K to 1100 K above which it shows the normal positive thermal expansion; furthermore, the material has cubic symmetry over this entire temperature range, and up to at least 1600 K at ambient pressure.
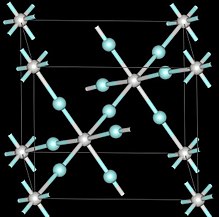
[6] At ambient pressures scandium trifluoride adopts the cubic crystal system, using the perovskite structure with one metal position vacant.
[8][9] It exhibits nonlinear optical properties for frequency conversion and can luminesce when doped with rare-earth ions.