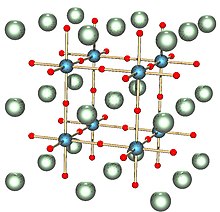
Perovskite (structure)
[2] The mineral was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856).
In addition to being one of the most abundant structural families, perovskites wide-ranging properties and applications.
The relative ion size requirements for stability of the cubic structure are quite stringent, so slight buckling and distortion can produce several lower-symmetry distorted versions, in which the coordination numbers of A cations, B cations or both are reduced.
Conversely, off-centering of an undersized B cation within its octahedron allows it to attain a stable bonding pattern.
The resulting electric dipole is responsible for the property of ferroelectricity and shown by perovskites such as BaTiO3 that distort in this fashion.
The prime example is yttrium barium copper oxide (YBCO), which has the formula YBa2Cu3O7.
When heated in the absence of O2, the solid loses its superconducting properties, relaxes to the stoichiometry YBa2Cu3O6.5, and all copper sites convert to Cu2+.
[16][17] Octahedral tilting can occur in double perovskites, however Jahn–Teller distortions and alternative modes alter the B–O bond length.
Beyond the most common perovskite symmetries (cubic, tetragonal, orthorhombic), a more precise determination leads to a total of 23 different structure types that can be found.
[18] These 23 structure can be categorized into 4 different so-called tilt systems that are denoted by their respective Glazer notation.
At high pressures associated with the deeper mantel, the Si sites feature octahedral units.
However, it cannot be transported from depths of several hundred km to the Earth's surface without transforming back into less dense materials.
MgCNi3 is a metallic perovskite compound and has received lot of attention because of its superconducting properties.
Physical properties of interest to materials science among perovskites They are applicable to lasers.
[30] The financially biggest application of perovskites is in ceramic capacitors, in which BaTiO3 is used because of its high dielectric constant.
[31][32] Light-emitting diodes exploit the high photoluminescence quantum efficiencies of perovskites.
[35][36] Scintillators based on cerium-doped lutetium aluminum perovskite (LuAP:Ce) single crystals were reported.