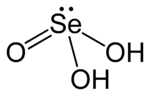
Selenous acid
In solution it is a diprotic acid:[3] It is moderately oxidizing in nature, but kinetically slow.
[6] Another use for selenious acid is the chemical darkening and patination of copper, brass and bronze, producing a rich dark brown color that can be further enhanced with mechanical abrasion.
[7] Selenious acid is a key component of the Mecke reagent used for drug checking.
[8][9] Selenous acid can supply the trace element indicated in people as a source of selenium.
[10][11] Like many selenium compounds, selenous acid is highly toxic in excessive quantities, and ingestion of any significant quantity of selenous acid is usually fatal, however it is an approved dietary source in proper amounts.