Selenium oxydichloride
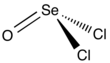
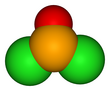
Selenium oxydichloride is the inorganic compound with the formula SeOCl2.
Structurally, it is a close chemical relative of thionyl chloride SOCl2, being a pyramidal molecule.
Pure selenium oxydichloride autoionizes to a dimer:[4] The SeOCl2 is generally a labile Lewis acid and solutions of sulfur trioxide in SeOCl2 likely form [SeOCl]+[SO3Cl]− the same way.
[5] The compound hydrolyzes readily to form hydrogen chloride and selenium dioxide,[citation needed] and very few organic compounds dissolve in it without reaction.
At elevated temperatures, it is a strong oxidizer, yielding a chloride, selenium dioxide, and diselenium dichloride.