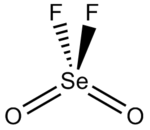
Selenoyl fluoride
Selenoyl fluoride, selenoyl difluoride, selenium oxyfluoride, or selenium dioxydifluoride is a chemical compound with the formula SeO2F2.
[3] The Se-F bond length is 1.685 Å and the selenium to oxygen bond is 1.575 Å long.
[4] Selenoyl fluoride can be formed by the action of warm fluorosulfonic acid on barium selenate[5] or selenic acid.
It may react violently upon contact with ammonia.
Selenoyl fluoride reacting with xenon difluoride gives FXeOSeF5.