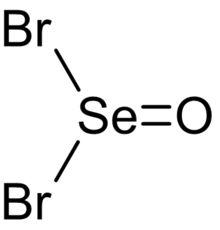
Selenium oxybromide
Dissolving the selenium dioxide in the tetrabromide will produce the oxybromide.
[3] Evidence from infrared and polarized Raman spectroscopy suggests that selenium oxybromide adopts a pyramidal molecular geometry with Cs symmetry,[4] like other chalcogen(IV) oxohalides such as thionyl bromide (SOBr2) and selenium oxydichloride (SeOCl2).
Its electrical conductivity in the liquid state just above the melting temperature is 6×10−5 S/m.
SeOBr2 is highly reactive, with most reactions taking place in the liquid state.
Iron, copper, gold, platinum, and zinc are all attacked by SeOBr2.