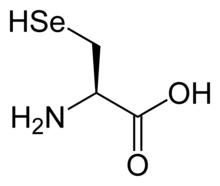
Selenocysteine
Selenocysteine (symbol Sec or U,[4] in older publications also as Se-Cys)[5] is the 21st proteinogenic amino acid.
Like other natural proteinogenic amino acids, cysteine and selenocysteine have L chirality in the older D/L notation based on homology to D- and L-glyceraldehyde.
[16] When cells are grown in the absence of selenium, translation of selenoproteins terminates at the UGA codon, resulting in a truncated, nonfunctional enzyme.
The SECIS element is defined by characteristic nucleotide sequences and secondary structure base-pairing patterns.
In bacteria, the SECIS element is typically located immediately following the UGA codon within the reading frame for the selenoprotein.
[17] In Archaea and in eukaryotes, the SECIS element is in the 3′ untranslated region (3′ UTR) of the mRNA and can direct multiple UGA codons to encode selenocysteine residues.
The primary and secondary structure of selenocysteine-specific tRNA, tRNASec, differ from those of standard tRNAs in several respects, most notably in having an 8-base-pair (bacteria) or 10-base-pair (eukaryotes)[Archaea?]
acceptor stem, a long variable region arm, and substitutions at several well-conserved base positions.
[23] Selenocysteine derivatives γ-glutamyl-Se-methylselenocysteine and Se-methylselenocysteine occur naturally in plants of the genera Allium and Brassica.