Self-assembled monolayer
[7] At higher molecular coverage, adsorbates can begin to form three-dimensional crystalline or semicrystalline structures on the substrate surface over a period of minutes to hours.
Areas of close-packed molecules nucleate and grow until the surface of the substrate is covered in a single monolayer.
[citation needed] Thiol-metal bonds are on the order of 100 kJ/mol, making them fairly stable in a variety of temperatures, solvents, and potentials.
[10] Monolayers pack tightly due to van der Waals interactions,[1][12] thereby reducing their own free energy.
Interest in such dithiols stems from the possibility of linking the two sulfur ends to metallic contacts, which was first used in molecular conduction measurements.
Self-assembled monolayers of thiolates on noble metals are a special case because the metal-metal bonds become reversible after the formation of the thiolate-metal complex.
[17] This reversibility is what gives rise to vacancy islands and it is why SAMs of alkanethiolates can be thermally desorbed and undergo exchange with free thiols.
[20][21] Special attention is essential in some cases, such as that of dithiol SAMs to avoid problems due to oxidation or photoinduced processes, which can affect terminal groups and lead to disorder and multilayer formation.
[22][23] In this case appropriate choice of solvents, their degassing by inert gasses and preparation in the absence of light is crucial[22][23] and allows formation of "standing up" SAMs with free –SH groups.
This method has also been used to give information on relative binding strengths of SAMs with different head groups and more generally on self-assembly characteristics.
[18][25] The thicknesses of SAMs can be measured using ellipsometry and X-ray photoelectron spectroscopy (XPS), which also give information on interfacial properties.
AFM has been used to determine chemical functionality, conductance, magnetic properties, surface charge, and frictional forces of SAMs.
An alternative characterisation instrument for measuring the self-assembly in real time is dual polarisation interferometry where the refractive index, thickness, mass and birefringence of the self assembled layer are quantified at high resolution.
Another method that can be used to measure the self-assembly in real-time is Quartz Crystal Microbalance with Dissipation monitoring technology where the mass and viscoelastic properties of the adlayer are quantified.
[20][21] The kinetics of adsorption and temperature induced desorption as well as information on structure can also be obtained in real time by ion scattering techniques such as low energy ion scattering (LEIS) and time of flight direct recoil spectroscopy (TOFDRS).
[1][10] SAMs intrinsically form defects due to the thermodynamics of formation, e.g. thiol SAMs on gold typically exhibit etch pits (monatomic vacancy islands) likely due to extraction of adatoms from the substrate and formation of adatom-adsorbate moieties.
SAMs on nanoparticles, including colloids and nanocrystals, "stabilize the reactive surface of the particle and present organic functional groups at the particle-solvent interface".
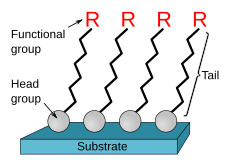
[1] These organic functional groups are useful for applications, such as immunoassays or sensors, that are dependent on chemical composition of the surface.
[7] Dual polarisation interferometry being a real time technique with ~10 Hz resolution can measure the kinetics of monolayer self-assembly directly.
Similar to nucleation in metals, as these islands grow larger they intersect forming boundaries until they end up in phase 3, as seen below.
In the first path the heads of the SAM organize to their near final locations with the tail groups loosely formed on top.
To minimize the free energy of the organic layer the molecules adopt conformations that allow high degree of Van der Waals forces with some hydrogen bonding.
In typical applications α varies from 0 to 60 degrees depending on the substrate and type of SAM molecule.
In general, however, the kinetics are dependent on both preparations conditions and material properties of the solvent, adsorbate and substrate.
[1] This first strategy involves locally depositing self-assembled monolayers on the surface only where the nanostructure will later be located.
The major techniques that use this strategy are:[33] SAMs are an inexpensive and versatile surface coating for applications including control of wetting and adhesion,[36] chemical resistance, bio compatibility, sensitization, and molecular recognition for sensors[37] and nano fabrication.
A common household product, Rain-X, utilizes SAMs to create a hydrophobic monolayer on car windshields to keep them clear of rain.
The terminal group is then modified to attract a specific material like a particular nanoparticle, wire, ribbon, or other nanostructure.
The ability to pattern these SAMs allows them to be placed in configurations that increase sensitivity and do not damage or interfere with other components of the biosensor.