Self-ionization of water
The hydrogen nucleus, H+, immediately protonates another water molecule to form a hydronium cation, H3O+.
The self-ionization of water was first proposed in 1884 by Svante Arrhenius as part of the theory of ionic dissociation which he proposed to explain the conductivity of electrolytes including water.
At that time, nothing was yet known of atomic structure or subatomic particles, so he had no reason to consider the formation of an
Later spectroscopic evidence has shown that many protons are actually hydrated by more than one water molecule.
, where aq (for aqueous) indicates an indefinite or variable number of water molecules.
The ions are produced by the water self-ionization reaction, which applies to pure water and any aqueous solution: Expressed with chemical activities a, instead of concentrations, the thermodynamic equilibrium constant for the water ionization reaction is: which is numerically equal to the more traditional thermodynamic equilibrium constant written as: under the assumption that the sum of the chemical potentials of H+ and H3O+ is formally equal to twice the chemical potential of H2O at the same temperature and pressure.
[1] Because most acid–base solutions are typically very dilute, the activity of water is generally approximated as being equal to unity, which allows the ionic product of water to be expressed as:[2] In dilute aqueous solutions, the activities of solutes (dissolved species such as ions) are approximately equal to their concentrations.
When the equilibrium constant is written as a product of concentrations (as opposed to activities) it is necessary to make corrections to the value of
For many practical purposes, the molality (mol solute/kg water) and molar (mol solute/L solution) concentrations can be considered as nearly equal at ambient temperature and pressure if the solution density remains close to one (i.e., sufficiently diluted solutions and negligible effect of temperature changes).
The main advantage of the molal concentration unit (mol/kg water) is to result in stable and robust concentration values which are independent of the solution density and volume changes (density depending on the water salinity (ionic strength), temperature and pressure); therefore, molality is the preferred unit used in thermodynamic calculations or in precise or less-usual conditions, e.g., for seawater with a density significantly different from that of pure water,[3] or at elevated temperatures, like those prevailing in thermal power plants.
This is analogous to the notations pH and pKa for an acid dissociation constant, where the symbol p denotes a cologarithm.
The logarithmic form of the equilibrium constant equation is pKw = pH + pOH.
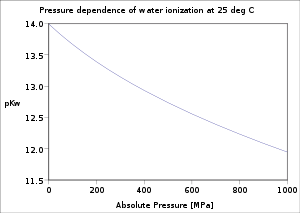
The dependence of the water ionization on temperature and pressure has been investigated thoroughly.
Example values for superheated steam (gas) and supercritical water fluid are given in the table.
Heavy water, D2O, self-ionizes less than normal water, H2O; This is due to the equilibrium isotope effect, a quantum mechanical effect attributed to oxygen forming a slightly stronger bond to deuterium because the larger mass of deuterium results in a lower zero-point energy.
[9] In water–heavy water mixtures equilibria several species are involved: H2O, HDO, D2O, H3O+, D3O+, H2DO+, HD2O+, HO−, DO−.
The following sequence of events has been proposed on the basis of electric field fluctuations in liquid water.
[10] Random fluctuations in molecular motions occasionally (about once every 10 hours per water molecule[11]) produce an electric field strong enough to break an oxygen–hydrogen bond, resulting in a hydroxide (OH−) and hydronium ion (H3O+); the hydrogen nucleus of the hydronium ion travels along water molecules by the Grotthuss mechanism and a change in the hydrogen bond network in the solvent isolates the two ions, which are stabilized by solvation.
Within 1 picosecond, however, a second reorganization of the hydrogen bond network allows rapid proton transfer down the electric potential difference and subsequent recombination of the ions.
This timescale is consistent with the time it takes for hydrogen bonds to reorientate themselves in water.
[15] Water molecules dissociate into equal amounts of H3O+ and OH−, so their concentrations are almost exactly 1.00×10−7 mol dm−3 at 25 °C and 0.1 MPa.
The concentration of OH− will decrease in such a way that the product [H3O+][OH−] remains constant for fixed temperature and pressure.