Serine protease
Serine proteases fall into two broad categories based on their structure: chymotrypsin-like (trypsin-like) or subtilisin-like.
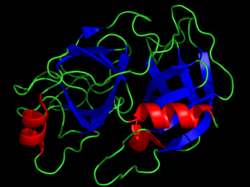
Serine proteases are characterised by a distinctive structure, consisting of two beta-barrel domains that converge at the catalytic active site.
[5] Trypsin-like proteases cleave peptide bonds following a positively charged amino acid (lysine or arginine).
This results in a specificity for medium to large sized hydrophobic residues, such as tyrosine, phenylalanine and tryptophan.
They have been found to have roles in coagulation and digestion as well as in the pathophysiology of neurodegenerative disorders such as Alzheimer's and Parkinson's induced dementia.
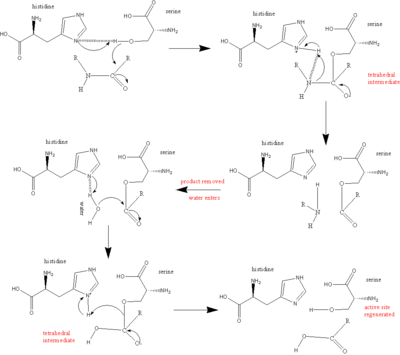
The triad is a coordinated structure consisting of three amino acids: His 57, Ser 195 (hence the name "serine protease") and Asp 102.
These three key amino acids each play an essential role in the cleaving ability of the proteases.
While the amino acid members of the triad are located far from one another on the sequence of the protein, due to folding, they will be very close to one another in the heart of the enzyme.
In effect, serine proteases preferentially bind the transition state and the overall structure is favored, lowering the activation energy of the reaction.
Acute pancreatitis is such a condition, in which there is premature activation of the digestive enzymes in the pancreas, resulting in self-digestion (autolysis).
Zymogens are large, inactive structures, which have the ability to break apart or change into the smaller activated enzymes.
Exogenous snake venom serine proteases cause a vast array of coagulopathies when injected in a host due to the lack of regulation of their activity.
Due to their catalytic activity, some serine proteases possess potent antimicrobial properties.
Several in vitro studies have demonstrated the efficacy of some proteases in reducing virulence by cleaving viral surface proteins.