Silicon nitride
Si3N4 (Trisilicon tetranitride) is the most thermodynamically stable and commercially important of the silicon nitrides,[6] and the term ″Silicon nitride″ commonly refers to this specific composition.
Without an iron catalyst, the reaction is complete after several hours (~7), when no further weight increase due to nitrogen absorption (per gram of silicon) is detected.
[citation needed] In addition to Si3N4, several other silicon nitride phases (with chemical formulas corresponding to varying degrees of nitridation/Si oxidation state) have been reported in the literature.
The diimide decomposition results in amorphous silicon nitride, which needs further annealing under nitrogen at 1400–1500 °C to convert it to a crystalline powder; this is now the second-most-important route for commercial production.
[16] A cleaner alternative is to use spark plasma sintering, where heating is conducted very rapidly (seconds) by passing pulses of electric current through the compacted powder.
Due to the c-glide plane that relates AB to CD, the α structure contains cavities instead of tunnels.
Growth is estimated at 40% per year, but could be even higher if ceramic bearings are selected for consumer applications such as in-line skates and computer disk drives.
To demonstrate this capability in a complex configuration, NASA scientists used advanced rapid prototyping technology to fabricate a one-inch-diameter, single-piece combustion chamber/nozzle (thruster) component.
[32] Silicon nitride was used for the "microshutters" developed for the Near Infrared Spectrograph aboard the James Webb Space Telescope.
[34][35] The material is also an alternative to PEEK (polyether ether ketone) and titanium, which are used for spinal fusion devices (with latter being relatively expensive).
[36][37] It is silicon nitride's hydrophilic, microtextured surface that contributes to the material's strength, durability and reliability compared to PEEK and titanium.
Bulk, monolithic silicon nitride is used as a material for cutting tools, due to its hardness, thermal stability, and resistance to wear.
Hot hardness, fracture toughness and thermal shock resistance mean that sintered silicon nitride can cut cast iron, hard steel and nickel based alloys with surface speeds up to 25 times quicker than those obtained with conventional materials such as tungsten carbide.
For example, face milling of gray cast iron with silicon nitride inserts doubled the cutting speed, increased tool life from one part to six parts per edge, and reduced the average cost of inserts by 50%, as compared to traditional tungsten carbide tools.
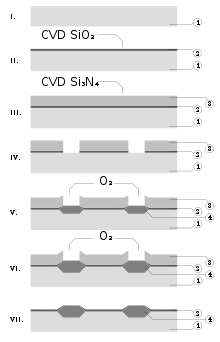
[9][27] Silicon nitride is often used as an insulator and chemical barrier in manufacturing integrated circuits, to electrically isolate different structures or as an etch mask in bulk micromachining.
As a passivation layer for microchips, it is superior to silicon dioxide, as it is a significantly better diffusion barrier against water molecules and sodium ions, two major sources of corrosion and instability in microelectronics.
They are plasma deposited using the following reactions:[10] These SiNH films have much less tensile stress, but worse electrical properties (resistivity 106 to 1015 Ω·cm, and dielectric strength 1 to 5 MV/cm),[10][43] and are thermally stable to high temperatures under specific physical conditions.
Silicon Nitride photonic integrated circuits have a broad spectral coverage and features low light losses.
[49] Silicon nitride has emerged as a favorable platform for high-stress thin film membrane devices.
[53] The first synthesis of silicon nitride was reported in 1857 by Henri Etienne Sainte-Claire Deville and Friedrich Wöhler.
Paul Schuetzenberger first reported a product with the composition of the tetranitride, Si3N4, in 1879 that was obtained by heating silicon with brasque (a paste made by mixing charcoal, coal, or coke with clay which is then used to line crucibles) in a blast furnace.
From 1948 to 1952, the Carborundum Company, Niagara Falls, New York, applied for several patents on the manufacture and application of silicon nitride.
[9] By 1958 Haynes (Union Carbide) silicon nitride was in commercial production for thermocouple tubes, rocket nozzles, and boats and crucibles for melting metals.
In 1971, the Advanced Research Project Agency of the US Department of Defense placed a US$17 million contract with Ford and Westinghouse for two ceramic gas turbines.