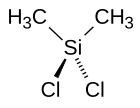
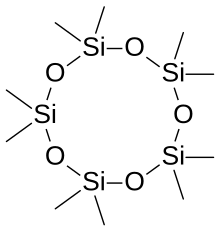
Siloxane
Siloxanes are manmade and have many commercial and industrial applications because of the compounds’ hydrophobicity, low thermal conductivity, and high flexibility.
This geometric consideration is the basis of the useful properties of some siloxane-containing materials, such as their low glass transition temperatures.
Starting from trisilanols, cages are possible, such as the species with the formula (RSi)nO3n/2 with cubic (n = 8) and hexagonal prismatic (n = 12) structures.
The cubic cages are cubane-type clusters, with silicon centers at the corners of a cube oxygen centres spanning each of the twelve edges.
This conversion is illustrated by the combustion of hexamethylcyclotrisiloxane: Strong base degrades siloxane group, often affording siloxide salts: This reaction proceeds by production of silanols.
By exploiting this reaction, polysiloxanes have been used as preceramic polymers in various processes including additive manufacturing.
Cyclomethicones are a group of methyl siloxanes, a class of liquid silicones (cyclic polydimethylsiloxane polymers) that possess the characteristics of low viscosity and high volatility as well as being skin emollients and in certain circumstances useful cleaning solvents.
[7] Unlike dimethicones, which are linear siloxanes that do not evaporate, cyclomethicones are cyclic: both groups consist of a backbone of [(CH3)2SiO]n. They are used in many cosmetic products including deodorants and antiperspirants which need to coat the skin but not remain tacky afterward.
[11] Even though some cyclomethicones structurally resemble crown ethers, they bind metal ions only weakly.