Sodium hexanitritocobaltate(III)
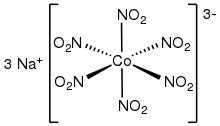
Sodium hexanitritocobaltate(III) is an inorganic compound with the formula Na3[Co(NO2)6].
The anion of this yellow-coloured salt consists of the transition metal nitrite complex [Co(NO2)6]3−.
It was a reagent for the qualitative test for potassium and ammonium ions.
[2] The compound is prepared by oxidation of cobalt(II) salts in the presence of sodium nitrite:[3] Although the sodium cobaltinitrite is soluble in water, it forms the basis of a quantitative determination of potassium, thallium, and ammonium ions.
Under the recommended reaction conditions the insoluble double salt, K2Na[Co(NO2)6]·H2O is precipitated and weighed.