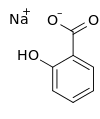
Sodium salicylate
It can be prepared from sodium phenolate and carbon dioxide under higher temperature and pressure.
Historically, it has been synthesized by refluxing methyl salicylate (wintergreen oil) with an excess of sodium hydroxide.
It is a shiny white powder with an aromatic taste.
[6] Sodium salicylate also acts as non-steroidal anti-inflammatory drug (NSAID), and induces apoptosis in cancer cells [7][8][9] and also necrosis.
[10] It is also a potential replacement for aspirin for people sensitive to it.