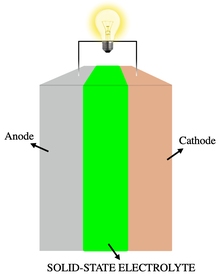
Solid-state electrolyte
[6] Despite the promising advantages, there are still many limitations that are hindering the transition of SSEs from academia research to large-scale production, depending mainly on the poor ionic conductivity compared to that of liquid counterparts.
However, many car OEMs (Toyota, BMW, Honda, Hyundai) expect to integrate these systems into viable devices and to commercialize solid-state battery-based electric vehicles by 2025.
[7][8] The first inorganic solid-state electrolytes were discovered by Michael Faraday in the nineteenth century, these being silver sulfide (Ag2S) and lead(II) fluoride (PbF2).
[10][11] However, unresolved fundamental issues remain in order to fully understand the behavior of all-solid batteries, especially in the area of electrochemical interfaces.
On the other hand, a QSSE, also called gel polymer electrolyte (GPE), is a freestanding membrane that contains a certain amount of liquid component immobilized inside the solid matrix.
[26] They are generally brittle and with this comes a low compatibility and stability towards the electrode, with a rapidly increasing interfacial resistance and a complicated scale-up from academic to industry.
Moreover, they possess higher elasticity and plasticity giving stability at the interface, flexibility and improved resistance to volume changes during operation.
The main alternatives to polyether-based SPEs are polycarbonates,[45] polyesters,[46] polynitriles (e.g. PAN),[47] polyalcohols (e.g. PVA),[48] polyamines (e.g. PEI),[49] polysiloxane (e.g. PDMS)[50][51] and fluoropolymers (e.g. PVDF, PVDF-HFP).
[52] Bio-polymers like lignin,[53] chitosan[54] and cellulose[55] are also gaining a lot of interest as standalone SPEs or blended with other polymers, on one side for their environmentally friendliness and on the other for their high complexation capability on the salts.
[62] Copolymerization,[63] crosslinking,[64] interpenetration,[65] and blending[66] may also be used as polymer/polymer coordination to tune the properties of the SPEs and achieve better performances, introducing in the polymeric chains polar groups like ethers, carbonyls or nitriles drastically improve the dissolution of the lithium salts.
This liquid electrolyte serves as a percolating pathway of ion conduction while the solid matrix adds mechanical stability to the material as a whole.
[72][73][74] Low molecular weight poly(ethylene glycol) (PEG) or other ethers or aprotic organic solvents with high dielectric constant like dimethylsulfoxide (DMSO) can also be mixed the SPE matrix.
[75][76] UV and thermal cross-linking are useful ways to polymerize in-situ the GPE directly in contact with the electrodes for a perfectly adherent interface.
[85] The usage of a SSE guarantees a homogeneous contact with the metallic lithium electrode and possess the mechanical properties to impede the uncontrolled deposition of Li+ ions during the charging phase.