Solubility equilibrium
The solid may dissolve unchanged, with dissociation, or with chemical reaction with another constituent of the solution, such as acid or alkali.
A solubility product has a similar functionality to an equilibrium constant though formally Ksp has the dimension of (concentration)p+q.
It occurs because solubility products, like other types of equilibrium constants, are functions of temperature.
In accordance with Le Chatelier's Principle, when the dissolution process is endothermic (heat is absorbed), solubility increases with rising temperature.
This effect is the basis for the process of recrystallization, which can be used to purify a chemical compound.
When dissolution is exothermic (heat is released) solubility decreases with rising temperature.
[3] This differential expression for a non-electrolyte can be integrated on a temperature interval to give:[4]
In gravimetric analysis for silver, the reduction in solubility due to the common ion effect is used to ensure "complete" precipitation of AgCl.
Solubility will increase with decreasing size of solute particle (or droplet) because of the additional surface energy.
For example, aragonite and calcite will have different solubility products even though they have both the same chemical identity (calcium carbonate).
However, kinetic factors may favor the formation the unfavorable precipitate (e.g. aragonite), which is then said to be in a metastable state.
Amorphous drugs have higher solubility than their crystalline counterparts due to the absence of long-distance interactions inherent in crystal lattice.
[8][9] For condensed phases (solids and liquids), the pressure dependence of solubility is typically weak and usually neglected in practice.
This is equivalent to defining the standard state as the saturated solution so that the activity coefficient is equal to one.
Sucrose is unusual in that it does not easily form a supersaturated solution at higher concentrations, as do most other carbohydrates.
When the solubility of the salt is very low the activity coefficients of the ions in solution are nearly equal to one.
However, general-purpose computer programs are designed to use hydrogen ion concentrations with the alternative definitions.
A typical reaction with dissolution involves a weak base, B, dissolving in an acidic aqueous solution.
[14] Dissolution of weak acids in alkaline media is similarly important.
Leaching of aluminium salts from rocks and soil by acid rain is another example of dissolution with reaction: alumino-silicates are bases which react with the acid to form soluble species, such as Al3+(aq).
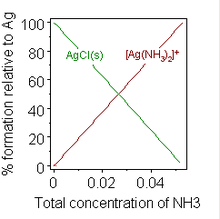
A well-known example is the addition of a concentrated solution of ammonia to a suspension of silver chloride, in which dissolution is favoured by the formation of an ammine complex.
When sufficient ammonia is added to a suspension of silver chloride, the solid dissolves.
The addition of water softeners to washing powders to inhibit the formation of soap scum provides an example of practical importance.
In static methods a mixture is brought to equilibrium and the concentration of a species in the solution phase is determined by chemical analysis.
[15] Very low concentrations can be measured if a radioactive tracer is incorporated in the solid phase.
A variation of the static method is to add a solution of the substance in a non-aqueous solvent, such as dimethyl sulfoxide, to an aqueous buffer mixture.
The cloudiness is due to the fact that the precipitate particles are very small resulting in Tyndall scattering.
Over time the cloudiness will disappear as the size of the crystallites increases, and eventually equilibrium will be reached in a process known as precipitate ageing.
Subsequently, the rate of change of pH due to precipitation or dissolution is monitored and strong acid and base titrant are added to adjust the pH to discover the equilibrium conditions when the two rates are equal.
The advantage of this method is that it is relatively fast as the quantity of precipitate formed is quite small.