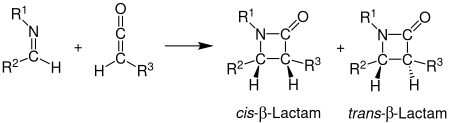
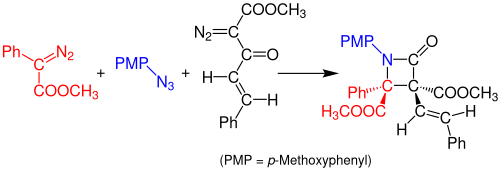
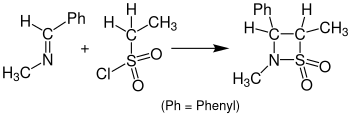
Staudinger synthesis
The first step is a nucleophilic attack by the imine nitrogen on the carbonyl carbon to generate a zwitterionic intermediate.
Under photochemical and microwave conditions the intermediate's 4π-electron system cannot undergo a disrotatory ring closure to form the β-lactam, possibly because the two double bonds are not coplanar.
[12] If the ring closure step is rate-determining, stereochemical predictions based on torquoselectivity are reliable.
The ketene substituent affects the transition state by either speeding up or slowing down the progress towards the β-lactam.
[11] Reviews on asymmetric induction of the Staudinger synthesis, including the use of organic and organometallic catalysts, have been published.
In 2014, Doyle and coworkers reported a one-pot, multicomponent Staudinger synthesis of β-lactams from azides and two diazo compounds.
The solvent used for this reaction is dichloromethane (DCM) and the solution needs to rest for 3 hours at room temperature.