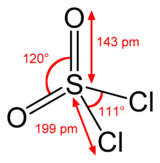
Sulfuryl chloride
SO2Cl2 is prepared by the reaction of sulfur dioxide and chlorine in the presence of a catalyst, such as activated carbon.
Sulfuryl chloride was first prepared in 1838 by the French chemist Henri Victor Regnault.
Upon standing, SO2Cl2 decomposes to sulfur dioxide and chlorine, which gives the older samples a slightly yellowish color.
Sulfuryl chloride is used in the conversion of C−H to C−Cl adjacent to activating substituents such as carbonyls and sulfoxides:[5][6] It also chlorinates alkanes, alkenes, alkynes, aromatics, ethers (such as tetrahydrofuran) and epoxides.
It releases hydrogen chloride upon contact with water, as well as donor solvents such as dimethyl sulfoxide and dimethylformamide.