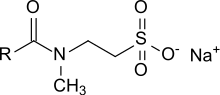
Taurates
Taurates rapidly spread due to their lime resistance and their oil-removing effect in textile treatment, as detergent raw material and in cosmetics applications.
The production of taurates decreased after the outbreak of the World War II, since only poor quality fatty acids were available due to the fat management.
[1] Taurates were first obtained by the Schotten-Baumann method which is the reaction of long-chain carboxylic acid chlorides with aqueous solutions of the sodium salt of N-methyltaurine.
Therefore, more recent variants of the direct amidation aim at gentler process conditions using suitable catalysts, such as sodium borohydride,[5] boric acid or zinc oxide.
Taurates are used as mild, well-foaming surfactants in body cleansing and personal care products (shampoos, liquid soaps and cleansers, face lotions, skin creams, bubble baths, syndet soaps), textile processing (wetting agents and detergents, dye dispersants), in crop protection formulations and in other industrial applications.