Terminal deoxynucleotidyl transferase
The diversity introduced by TdT has played an important role in the evolution of the vertebrate immune system, significantly increasing the variety of antigen receptors that a cell is equipped with to fight pathogens.
Studies using TdT knockout mice have found drastic reductions (10-fold) in T-cell receptor (TCR) diversity compared with that of normal, or wild-type, systems.
[7] In 2016–18, TdT was discovered to demonstrate in trans template dependant behaviour in addition to its more broadly known template independent behaviour[10][11] TdT is absent in fetal liver HSCs, significantly impairing junctional diversity in B-cells during the fetal period.
Further, TdT is the only polymerase that is known to catalyze the synthesis of 2-15nt DNA polymers from free nucleotides in solution in vivo.
[16] Cell lines derived from these patients served as one of the first sources of pure TdT and lead to the discovery that differences in activity exist between human and bovine isoforms.
[16] Similar to many polymerases, the catalytic site of TdT has two divalent cations in its palm domain that assist in nucleotide binding, help lower the pKa of the 3'-OH group and ultimately facilitate the departure of the resultant pyrophosphate by-product.
Additionally, TdTL reportedly can modulate the catalytic activity of TdTS in vivo through an unknown mechanism.
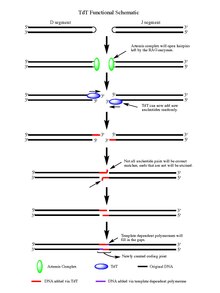
The hairpins are both opened by the Artemis complex, which has endonuclease activity when phosphorylated, providing the free 3' OH ends for TdT to act upon.
On average 2-5 random base pairs are added to each 3' end generated after the action of the Artemis complex.
Further, the discovery of this template dependant activity has led to more convincing mechanistic hypotheses as to how the distribution of lengths of the additions of the N regions arise in V(D)J recombination.
[30] TdT has also seen recent application in the De Novo synthesis of oligonucleotides, with TdT-dNTP tethered analogs capable of primer extension by 1 nt at a time.
[31] In other words, the enzyme TdT has demonstrated the capability of making synthetic DNA by adding one letter at a time to a primer sequence.