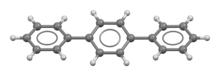
Terphenyl
Terphenyls are a group of closely related aromatic hydrocarbons.
Also known as diphenylbenzenes or triphenyls, they consist of a central benzene ring substituted with two phenyl groups.
Commercial grade terphenyl is generally a mixture of the three isomers.
This mixture is used in the production of polychlorinated terphenyls, which were formerly used as heat storage and transfer agents.
[2] p-Terphenyl derivatives are found in various fungi and bacteria.