Tertiary carbon
When a functional group is attached to a tertiary carbon, the prefix -tert (-t) is used in the common name for the compound.
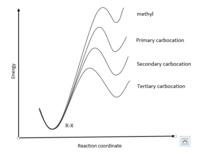
Tertiary carbons form the most stable carbocations due to a combination of factors.
The three alkyl groups on the tertiary carbon contribute to a strong inductive effect.
[7] In general, SN2 reactions do not occur with tertiary carbons because of the steric hindrance produced by the substituted groups.
However, recent research has shown there are exceptions to this rule; for the first time, a bimolecular nucleophilic substitution, aka SN2 reaction, can happen to a tertiary carbon.