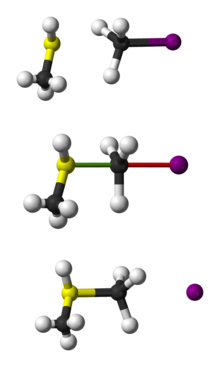
SN2 reaction
The bimolecular nucleophilic substitution (SN2) is a type of reaction mechanism that is common in organic chemistry.
The SN2 reaction can be considered as an organic-chemistry analogue of the associative substitution from the field of inorganic chemistry.
The reaction most often occurs at an aliphatic sp3 carbon center with an electronegative, stable leaving group attached to it, which is frequently a halogen (often denoted X).
[1] To achieve optimal orbital overlap, the nucleophile attacks 180° relative to the leaving group, resulting in the leaving group being pushed off the opposite side and the product formed with inversion of tetrahedral geometry at the central atom.
For SN2 reaction to occur more quickly, the nucleophile must easily access the sigma antibonding orbital between the central carbon and leaving group.
In SN2, however, the conjugation between the reaction centre and the adjacent pi system stabilizes the transition state.
tert-Butoxide, on the other hand, is a strong base, but a poor nucleophile, because of its three methyl groups hindering its approach to the carbon.
A good leaving group must be able to stabilize the electron density that comes from breaking its bond with the carbon center.
Leaving groups that are neutral, such as water, alcohols (R−OH), and amines (R−NH2), are good examples because of their positive charge when bonded to the carbon center prior to nucleophilic attack.
A polar aprotic solvent with low dielectric constant or a hindered dipole end will favour SN2 manner of nucleophilic substitution reaction.
Work with the 2-adamantyl system (SN2 not possible) by Schleyer and co-workers,[11] the use of azide (an excellent nucleophile but very poor leaving group) by Weiner and Sneen,[12][13] the development of sulfonate leaving groups (non-nucleophilic good leaving groups), and the demonstration of significant experimental problems in the initial claim of an SN1 mechanism in the solvolysis of optically active 2-bromooctane by Hughes et al.[14][3] have demonstrated conclusively that secondary substrates go exclusively (except in unusual but predictable cases) by the SN2 mechanism.
As steric hindrance around the electrophilic center increases, as with isobutyl bromide, substitution is disfavored and elimination is the predominant reaction.
A development attracting attention in 2008 concerns a SN2 roundabout mechanism observed in a gas-phase reaction between chloride ions and methyl iodide with a special technique called crossed molecular beam imaging.