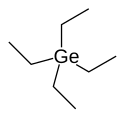
Tetraethylgermanium
Tetraethylgermanium (IUPAC name: tetraethylgermane), abbreviated TEG, is an organogermanium compound with the formula (CH3CH2)4Ge.
Tetraethylgermanium is an important chemical compound used in vapour deposition of germanium which is in a tetrahedral shape.
Clemens Winkler first reported the compound in 1887 from diethylzinc and germanium tetrachloride, shortly after germanium was discovered in 1887.
[1]