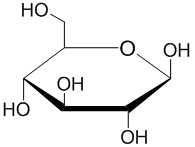
Pyranose
[2] Hermann Emil Fischer won the Nobel Prize in Chemistry (1902) for his work in determining the structure of the D-aldohexoses.
[1] However, the linear, free-aldehyde structures that Fischer proposed represent a very minor percentage of the forms that hexose sugars adopt in solution.
It was Edmund Hirst and Clifford Purves, in the research group of Walter Haworth, who conclusively determined that the hexose sugars preferentially form a pyranose, or six-membered, ring.
This puckering leads to a total of 38 distinct basic pyranose conformations: 2 chairs, 6 boats, 6 skew-boats, 12 half-chairs, and 12 envelopes.
Carbohydrate NMR takes advantage of these dihedral angles to determine the configuration of each of the hydroxyl groups around the ring.