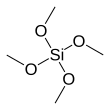
Tetramethyl orthosilicate
The basic properties are similar to the more popular tetraethyl orthosilicate, which is usually preferred because the product of hydrolysis, ethanol, is less toxic than methanol.
Tetramethyl orthosilicate hydrolyzes to SiO2: In organic synthesis, Si(OCH3)4 has been used to convert ketones and aldehydes to the corresponding ketals and acetals, respectively.
Worse, at low concentrations (200 ppm/15 min) the damage is often insidious, with onset of symptoms hours after exposure.
[3] The mode of action is the precipitation of silica in the eyes and/or lungs[citation needed].
Contrary to common information, including several erroneous MSDS sheets, the methanol produced is only a risk through chronic exposure and is a comparatively small concern.