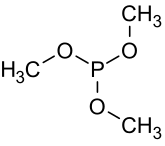
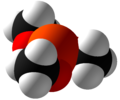
Trimethyl phosphite
It is the simplest phosphite ester and finds used as a ligand in organometallic chemistry and as a reagent in organic synthesis.
The molecule features a pyramidal phosphorus(III) center bound to three methoxy groups.
Trimethyl phosphite is in principle obtainable by methanolysis of phosphorus trichloride, say in the presence of a proton accepting base.
The formation of this ligand illustrates the susceptibility of trimethyl phosphite (and metal complexes thereof) to the Arbuzov reaction.
Trimethyl phosphite is also used as a mild desulfurization reagent in organic synthesis, for example in the preparation of derivatives of tetrathiafulvalene.