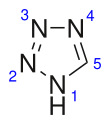
Tetrazole
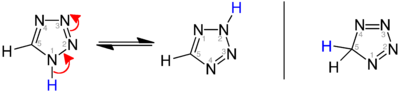
[8] Strongly inductively electron-withdrawing functional groups attached to a tetrazole may stabilize a tautomeric ring-opening equilibrium with an azidoimine form.
A Pinner reaction of organic nitriles with sodium azide in the presence of a buffered strong acid (e.g. triethylammonium chloride) synthesizes 5-substituted 1H-tetrazoles cleanly.
Tetrazoles can act as bioisosteres for carboxylate groups because they have similar pKa and are deprotonated at physiological pH.
[14] Studies suggest VT-1161 and VT-1129 are a potential potent antifungal drugs as they disturbs fungal enzymatic function but not human enzymes.
Tetrazole based energetic materials produce high-temperature, non-toxic reaction products such as water and nitrogen gas,[19] and have a high burn rate and relative stability,[20] all of which are desirable properties.