Heterocyclic compound
[2] Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes.
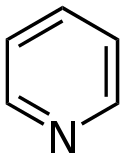
For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and furan are quinoline, benzothiophene, indole, and benzofuran, respectively.
Analogs of the previously mentioned heterocycles for this third family of compounds are acridine, dibenzothiophene, carbazole, and dibenzofuran, respectively.
In comparison with organic heterocycles, which have numerous commercial applications, inorganic ring systems are mainly of theoretical interest.
In a 7-membered ring, the heteroatom must be able to provide an empty π-orbital (e.g. boron) for "normal" aromatic stabilization to be available; otherwise, homoaromaticity may be possible.
For example, with the benzo-fused unsaturated nitrogen heterocycles, pyrrole provides indole or isoindole depending on the orientation.
Likewise, the compounds with two benzene rings fused to the central heterocycle are carbazole, acridine, and dibenzoazepine.
Some noteworthy developments:[10] Heterocyclic compounds are pervasive in many areas of life sciences and technology.