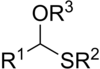
Thioacetal
In organosulfur chemistry, thioacetals are the sulfur (thio-) analogues of acetals (R−CH(−OR)2).
Dithioacetals generated from aldehydes and either 1,2-ethanedithiol or 1,3-propanedithiol are especially common among this class of molecules for use in organic synthesis.
As a result, dithioacetals can serve as protective groups for aldehydes.
Far from being unreactive, and in a reaction unlike that of aldehydes, that carbon can be deprotonated to render it nucleophilic: The inversion of polarity between R'(H)Cδ+=Oδ− and R'CLi(SR)2 is referred to as umpolung.
The lithiated intermediate can be used for various nucleophilic bond-forming reactions, and then the dithioketal hydrolyzed back to its carbonyl form.