Threonine ammonia-lyase
This enzyme was one of the first in which negative feedback inhibition by the end product of a metabolic pathway was directly observed and studied.
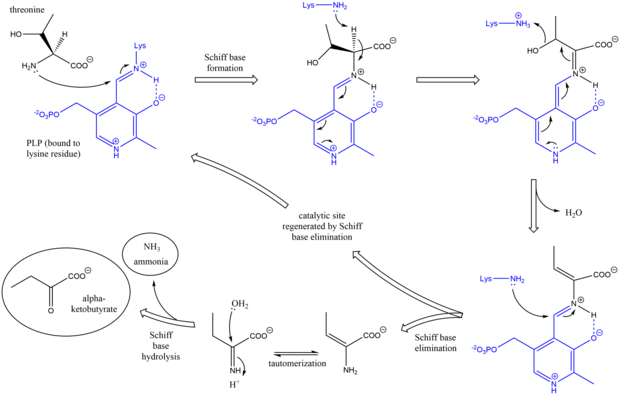
[5] While the threonine binding site is not perfectly understood, structural studies do reveal how the pyridoxal phosphate cofactor is bound.
After deprotonation of the amino acid alpha carbon and subsequent dehydration (hence the common name threonine dehydratase), a new Schiff base is formed.
This Schiff base is replaced by lysine attack, reforming the catalytically active PLP and releasing an initial alkene-containing product.
[8][9] After the final alpha-ketobutyrate product is generated, isoleucine is synthesized by progressing through the intermediates alpha-acetohydroxybutyrate to alpha-beta-dihydroxy-beta-methylvalerate, then to alpha-keto-beta-methylvalerate.
Valine promotes enzyme activity by competitively binding to the high affinity site, preventing isoleucine from having an inhibitory effect.
One is biosynthetic and resembles the enzyme characteristics presented here, while the other is degradative and functions to generate carbon fragments for energy production.
In plants, threonine ammonia-lyase is important in defense mechanisms against herbivores and is upregulated in response to abiotic stress.
[14] Other more exotic forms of the enzyme have been found that are extremely small in size, but still retain all catalytic and regulatory functions.