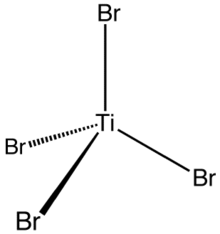
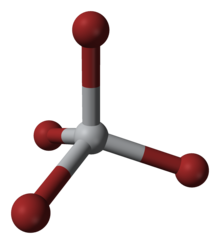
Titanium tetrabromide
Some key properties of these four-coordinated Ti(IV) species are their high Lewis acidity and their high solubility in nonpolar organic solvents.
It can be prepared via several methods: (i) from the elements, (ii) via the reaction of TiO2 with carbon and bromine (see Kroll process), and (iii) by treatment of TiCl4 with HBr.
[3] With bulky donor ligands, such as 2-methylpyridine (2-Mepy), five-coordinated adducts form.
[5] The tetrabromide and tetrachlorides of titanium react to give a statistical mixture of the mixed tetrahalides, TiBr4−xClx (x = 0-4).
[6] TiBr4 hydrolyzes rapidly, potentially dangerously, to release hydrogen bromide, otherwise known as hydrobromic acid.