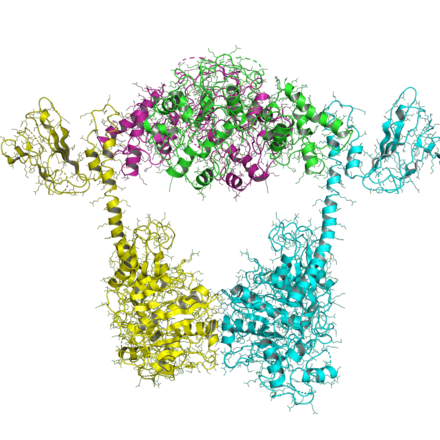Type II topoisomerase
Type II topoisomerases increase or decrease the linking number of a DNA loop by 2 units, and it promotes chromosome disentanglement.
For example, DNA gyrase, a type II topoisomerase observed in E. coli and most other prokaryotes, introduces negative supercoils and decreases the linking number by 2.
Gyrase and topoisomerase IV differ by their C-terminal domains, which is believed to dictate substrate specificity and functionality for these two enzymes.
In certain cancers, such as peripheral nerve sheath tumors, high expression of its encoded protein is also associated to poor patient survival.
The two classes of topoisomerases possess a similar strand passage mechanism and domain structure (see below), however they also have several important differences.
A coiled-coil region leads to a C-terminal domain that forms the main dimer interface for this crystal state (often termed the C-gate).
The topoisomerase II core was later solved in new conformations, including one by Fass et al.[14] and one by Dong et al.[15] The Fass structure shows that the Toprim domain is flexible and that this flexibility can allow the Toprim domain to coordinate with the WHD to form a competent cleavage complex.
This mechanism of bending resembles closely that of integration host factor (IHF) and HU, two architectural proteins in bacteria.
A recent structure of the topo VI A/B complex was solved, showing an open and closed conformation, two states that are predicted in the two-gate mechanism (see below).
A second strand of DNA, called the transport, or T-segment, is captured by the dimerization of the N-terminal ATPase domain (the ATPase-gate) when two molecules of ATP bind.
Hydrolysis of ATP and release of an inorganic phosphate leads to the cleavage of the G-segment, as the catalytic tyrosines form a covalent phosphotyrosine bond with the 5' end of the DNA.
Type IIB topoisomerases operate through a similar fashion, except that the protein forms a two-base overhang in the G-segment and the C-terminal gate is completely missing.
The mechanism of DNA cleavage by type IIA topoisomerases has recently been the focus of many biochemical and structural biology studies.
Linear DNA in eukaryotes is so long they can be thought of as being without ends; type II topoisomerases are needed for the same reason.
Due to their frequent presence in proliferating eukaryotic cells, inhibitors of type II topoisomerases have been extensively studied and used as anti-cancer medications.
While antibacterial compounds such as ciprofloxacin target bacterial gyrase, they fail to inhibit eukaryotic type IIA topoisomerases.
[citation needed] Recent structural studies have led to the discovery of a compound that no longer relies on this residue and, therefore, has efficacy against drug-resistant bacteria.
[citation needed] The bacteriophage (phage) T4 gyrase (type II topoismerase) is a multisubunit protein consisting of the products of genes 39, 52 and probably 60.
[32] Mutants defective in genes 39, 52 and 60 have reduced ability to carry out multiplicity reactivation, a form of recombinational repair that can deal with different types of DNA damage.
[33] The gyrase specified by the genome of uninfected E. coli also appears to participate in recombinational repair by providing an initiation point for the reciprocal strand exchange driven by the RecA protein.